You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
During the last decade, replacing missing teeth with implants has become a predictable treatment modality, providing functional and esthetic satisfaction. However, peri-implant tissue destruction sometimes occurs due to poor oral hygiene, resulting in the exposure of a few threads of the implant previously that were embedded in bone.1 The term ‘‘peri-implantitis’’ was introduced in the late 1980s and was subsequently defined as an inflammatory process affecting the soft and hard tissues around a functioning osseointegrated implant, resulting in the loss of supporting bone.2 It was so named because peri-implantitis microbiota is clinically, microbiologically, and histologically similar to microbiota of periodontitis.3
According to Karoussis et al,4 a significantly higher incidence of peri-implantitis was found in patients with a history of chronic periodontitis (28.6%) as compared with periodontally healthy subjects (5.8%). The most notable recent advances in the field of implantology have focused specifically on understanding the concepts of osseointegration and studying the structure of implants and their interaction with bone. Given the high number of implant placements each day around the world, a high prevalence of peri-implantitis can be anticipated, which underlines the necessity for a predictable therapy. In spite of diligent clinical trials and experimental studies, assiduous research is still required for the treatment of peri-implantitis, because there is still no standard protocol for its management. This article provides a comprehensive review of the studies published in national and international peer-reviewed literature published in English concerning the treatment of peri-implantitis.
Failing Implants
Broadly speaking, a failing implant demonstrates progressive loss of supporting bone structure but is clinically immobile, whereas a failed implant is clinically mobile or has spontaneously explanted.5,6 Implant failures can also be categorized as early or late. Early failures occur before osseointegration and prosthetic rehabilitation has taken place, while late failure occurs after the implant has been loaded with prosthesis. Late failures can also be subclassified as late-early or late-delayed.5,6 In failing implants, there is loss of supporting bone and mobility. Patients experience spontaneous pain as well as pain on clenching, percussion, or palpation, and deep pockets may be present.7
There are various methods available for the treatment of peri-implantitis, which include mechanical debridement, the use of antiseptics, adjunctive administration of local and/or systemic antibiotics, access flap surgery with or without the use of bone-regenerating procedures, and supportive therapy.8 Each treatment option has a window of effectiveness that seems to be defined primarily by initial probing pocket depth; in addition, certain methods for peri-implantitis treatment produce best results only within a given range of diagnostic parameters.9
Implant Maintenance and Mechanical Debridement
A patient with implants must follow proper protocols for their maintenance; this includes an annual visit to the dental office, where clinical and radiographic examinations are conducted to check for implant health and signs of peri-implantitis.10 Debridement is accomplished with implant-safe instruments. Plastic, graphite, and gold-tipped instruments can be used to remove deposits. An ultrasonic tip may be used only with a plastic covering that prevents gouging and disturbance of the titanium surface. Polishing the visible portion of the implant can be accomplished with rubber cups and nonabrasive polishing paste or tin oxide11 while scaling, which should be done with short working strokes and light pressure. Upon insertion of the instrument, the blade should be closed against the abutment and then opened past the deposit. The deposit should be engaged apically with the stroke extending coronally. A horizontal, oblique, or vertical stroke should be used, depending on the location of the deposit.12-15
Mechanical instrumentation to remove bacterial deposits may damage the implant surface if performed with metal instruments harder than titanium.16 According to a report by McCollum,17 a comparative study that evaluated the surface texture of titanium implant abutments after exposure to plastic scalers and an air–powder abrasive system or polishing with rubber cup and pumice found that none of these methods appeared to roughen the surface; a rubber cup with pumice provided the smoothest polished abutment surface.
Diagnosis
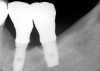
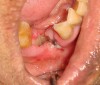
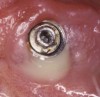
Radiographically, in peri-implantitis, vertical destruction of the crestal bone is present around the implant—which assumes the shape of a saucer—while the bottom part of the implant remains osseointegrated. In some instances, wedge-shaped defects develop along the implant (Figure 1). In addition, there is a peri-implant pocket and bleeding after gentle probing with a blunt instrument, and there may be suppuration from the pocket (Figure 2 and Figure 3). Tissues may or may not be swollen; however, hyperplasia is frequently seen if implants are located in an area with non-keratinized mucosa or if the suprastructure is an overdenture. Pain is not present.9 Both a mean loss of peri-implant bone height amounting to 1 mm to 1.5 mm in the first postsurgical year and vertical bone loss of less than 0.2 mm annually following the implant’s first year of service have been proposed as major criteria for success.18-20 It should be noted that peri-implant bone loss also occurs in cases of overload and faulty occlusion and may be be related to the type of implant used. The implants with the longest smooth surfaces demonstrated the highest amounts of bone resorption 12 months after abutment connection.21 Mobility of an implant suggests complete bone loss and, therefore, complete failure. To prevent this, peri-implant disease should be recognized earlier, to allow intervention before a substantial portion of the supporting bone is lost. However, mobility in early periods of osseointegration is not a very reliable clinical indicator of peri-implantitis. Therefore, electronic measuring devices should be used.
According to Mombelli et al,22 peri-implant probing depth measurements are more sensitive to force variation than the corresponding measurements around teeth. It has been suggested that probing of the implant sulcus is not truly diagnostic, and probing is indicated only in implants where pathology such as bleeding and exudate is present.23 Etter et al24 showed that although peri-implant probing disrupts the epithelial attachment to an implant surface, it does not cause permanent damage to the transmucosal seal, and completely new epithelial attachment is re-established within 5 days after peri-implant probing. Therefore, many authors suggest that probing around the implant should be done only when radiographic and clinical signs and symptoms are present. Successful implants generally allow probe penetration of approximately 3 mm; pockets of 5 mm or more signal peri-implantitis.9
If pockets of 3 mm are present, the patient’s oral hygiene should be improved, with more frequent recall visits. Pockets deeper than 3 mm with no bone loss call for improvements in oral hygiene and correction of unfavorable soft tissue. If pockets of 4 mm to 5 mm are present, cleaning of implants as well as correction of unfavorable soft tissue and use of antiseptic agents should be considered. In pockets deeper than 5 mm with moderate bone loss, treatment with local drug delivery is indicated. For pockets deeper than 5 mm with extensive bone loss, treatment with local drug delivery or surgical intervention should be considered (Table 1).9
Local Drug Delivery
Implant surfaces exposed to the oral cavity have rough surfaces, making elimination of infection difficult; as a result, adjunctive use of chemical antimicrobial agents has been advocated. Because peri-implant lesions are well demarked and contain the same microbiota as that of periodontitis, these agents kill bacteria effectively and have shown improvement in peri-implant lesions, which cannot be removed through mechanical debridement alone. In animal experiments, Ericsson et al25 showed that mechanical debridement—combined with systemic administration of amoxicillin and metronidazole—results in resolution of ligature-induced peri-implantitis lesions.
Topical chlorhexidine has been recommended for the treatment of early peri-implant infections. However, Porras et al26 found no difference in improvements following the use of topical chlorhexidine to supplement mechanical debridement compared to mechanical debridement alone; in addition, chlorhexidine gel showed only minor changes in treatment of peri-implantitis. In a clinical study by Mombelli and Lang,27 peri-implantitis lesions were mechanically debrided, pockets were irrigated with chlorhexidine, and adjunctive systemic administration of 1,000-mg ornidazole/day for 10 days was prescribed. This resulted in an improved clinical and microbiological condition for up to 12 months. Renvert et al28 used adjunctive minocycline microspheres, which resulted in improvements of both probing depth (PD) and bleeding on probing (BOP) scores. At the deepest peri-implant site, the mean PD decreased from 5 mm ± 0.9 mm to 4.1 mm ± 0.8 mm, and BOP scores were reduced from 100 mm ± 0% to 57 mm ± 35% after an observation period of 3 months. In another study, the same author repeatedly used local administration of minocycline microspheres 1 mg in addition to chlorhexidine gel 1%. Significant PD improvements were shown with minocycline microspheres as an adjunct compared to chlorhexidine alone at days 30, 90, and 180; and at the deepest sites of minocycline-treated implants, the mean PD reduction was 0.6 mm at 12 months.29
Salvi et al8 also used adjunctive local delivery of minocycline microspheres for treatment of peri-implantitis, and at the end of 12 months, he observed a decrease in mean PD from 5.9 mm ± 0.7 mm to 4.2 mm ± 0.6 mm, and BOP scores from 92% ± 28% to 44% ± 51%. This clinical study also demonstrated favorable results with the use of minocycline microspheres. Mombelli et al30 achieved comparable results with adjunctive local delivery of tetracycline-impregnated fibers after an observation period of 12 months, which showed PD reduction from 6.03 mm ± 1.54 mm to 3.85 mm ±1.49 mm at sites with the deepest probing pocket depth (PPD) at baseline. In this study, pathogens such as Tannerella forsythia, Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans were suppressed but they rebounded during the observation period.
Lasers in Peri-implantitis Treatment
Laser radiation has been expected to serve as an alternative or adjunctive treatment to conventional, mechanical periodontal therapy. Lasers provide various advantages, such as hemostatic effects, selective calculus ablation, and bactericidal effects. The interaction between laser light and metal surfaces is mainly determined by the degree of absorption and reflection. Each metal features a certain spectral reflection capacity, which is dependent on the specific wavelength of the laser. In the past, the use of lasers was contraindicated because the metal surfaces absorb the lasers’ wavelength, causing the potential for pitting, melting, and implant surface porosity. However, the lasers currently used in the treatment of peri-implantitis are reflected from the implant surface and cause the temperature in the bone to increase from 37°C to 47°C, which is the threshold for bone cells to remain viable. For example, the CO2 laser causes a temperature rise of only 3°C.31
Only the CO2 laser, diode laser, and Er:YAG laser are suitable for the irradiation of implant surfaces, as the implant body temperature does not increase significantly during irradiation.32-37 Also, these lasers showed bactericidal effects on textured implant surfaces in vitro. The Nd:YAG laser is not suitable for implant therapy, as it easily ablates the titanium, irrespective of output energy.33 Also, neither CO2 nor diode lasers were effective in removing plaque biofilms from root surfaces or titanium implants; thus, both types of lasers are used only adjunctively to mechanical treatment procedures.38-40
Diode lasers were used in a study by Bach et al,40 who found a significant improvement in the 5-year survival rate when integrating laser decontamination into the approved treatment protocol. Dortbudak et al41 found that the use of low-level laser therapy with a diode soft-tissue laser (690 nm) for 60 seconds after the placement of toluidine blue O for 1 minute on the contaminated surface reduced the counts of bacteria by a minimum of 92%. In another study, those authors were able to obtain complete bacterial elimination by using 905-nm diode (also a soft-tissue laser) with toluidine blue O on all types of implant surfaces (ie, machined, plasma-flame–sprayed, etched, and hydroxyapatite-coated).42 Mouhyi et al43 found that a combination of citric acid, hydrogen peroxide, and CO2 laser irradiation seems to be effective for cleaning and reestablishing the oxide structure of contaminated titanium surfaces. According to Schwarz et al,44 the Er:YAG laser was found to be effective in removing subgingival calculus from titanium implants without leading to any thermal damage. Kreisler et al,36 after evaluating 72 titanium blocks in vitro with three different surfaces, concluded that even at low energy densities, the Er:YAG laser has a high bactericidal potential on common implant surfaces, with no morphologic implant surface alterations detected.
In a study on Nd:YAG by Block et al,45 the authors found that that this wavelength did not sterilize the plasma-sprayed titanium or plasma-sprayed hydroxyapatite-coated titanium dental implants that were used in the study. In addition, melting, loss of porosity, and other surface alterations were observed on both types of implants, even at the lowest settings. Kreisler et al33 performed a study on various wavelengths including Nd:YAG, holmium:yttrium-aluminum-garnet (Ho:YAG), Er:YAG, CO2, and gallium-aluminum-arsenide for implant surface decontamination. They concluded that Nd:YAG and Ho:YAG lasers are not suitable for decontamination of dental implant surfaces at any power output. With Er:YAG and CO2, the power output must be limited so as to avoid surface damage.
Surgical Treatment of Peri-implantitis
Roos-Jansaker et al46 stated that it is difficult to remove biofilm from the implant surface—even using specially designed curettes and/or specially designed tips for ultrasonic devices—and that the surface structure of the implant contributes to the difficulties in the removal of hard and soft deposits from the implant surface without surgical intervention. However, according to Teughels et al,47 if the implant threads are exposed after healing following a surgical intervention, plaque retention will be facilitated and challenge the patient’s oral hygiene performance. Romeo et al48 therefore suggested the use of resective surgical approaches and smoothing of the implant surface as a treatment option; this treatment option reportedly affects the long-term survival of implants. Peri-implant bone defects with horizontal bone loss or craters with a narrow crestal opening may be more difficult to access for regenerative procedures.
Heitz-Mayfield and Lang49 clearly stated that prior to surgical therapy, the acute infection must be resolved and proper oral hygiene instituted. Mechanical debridement, therefore, is a must before the surgery. In addition to various devices already discussed are rotating single-use, disposable titanium debridement brushes. The advantages of using titanium brushes are short treatment time and more effective surface cleaning. According to Duddeck et al,50 SEM analyses of various implant surfaces demonstrates that stiff titanium bristles on rotating brushes allow for surface cleaning with only minor impact on all implant structures and are more gentle to the implant surface than other mechanical methods. The rotating single-use, disposable titanium debridement brushes are designed for angled and straight handpieces and should be used in conjunction with a rinse, such as pure water.
There are various methods available for decontamination of implants before the surgical procedure, and research demonstrates no difference between the degree of osseointegration of implants that were cleaned either with cotton pellets soaked in saline or with a rotating brush with pumice during regenerative surgery.51 Furthermore, Deppe et al52 could not detecte any difference between decontamination by a carbon dioxide laser and/or an air-powder abrasive unit during flap surgery with or without coverage of the defect by an e-PTFE membrane.
Air–powder abrasive units are often recommended for the surgical treatment of peri-implantitis. Most studies that evaluated the influence of various air–powder abrasive systems on the titanium surface noted increased implant surface roughness and retained powder particles, with no or only minor surface changes as a result of such application; in addition, the number of reported emphysema and pneumoparotitis cases induced by air–powder abrasive units appears to be low.53
Animal research has demonstrated that it is possible to obtain re-osseointegration on previously infected implants and to regenerate bone in experimentally created defects.1,54 Among human studies, Khoury and Buchmann55 reported an average bone fill of 1.7 mm to 2.5 mm when the authors used autogenous bone with or without the use of a resorbable or a non-resorbable membrane. However, the use of autogenous bone is not always advisable, as it involves an additional surgical site, thereby increasing patient discomfort. In a 6-month case series, Schwarz et al56 reported PD reductions and gains in clinical attachment levels using a bone substitute in combination with a resorbable membrane without submerged healing. Roos-Jansaker et al46 treated 36 peri-implantitis patients with bone loss of ≥ 3 threads as well as bleeding and/or pus on probing after the first year of healing. In all patients, the bone defects were filled with a bone substitute, fluorohydroxyapatite; among them, in 17 patients (Group 1), a resorbable membrane was placed over the grafted defect before suturing. One-year follow-up demonstrated a reduction in PDs of up to 2.9 mm in Group 1 and 3.4 mm in Group 2, whose defects were not covered with Osseoquest; the defect fill amounted to 1.5 mm and 1.4 mm.
Conclusion
The primary goals of treatment are to eliminate the inflammatory lesion, stop disease progression, and maintain the implant in function with healthy peri-implant tissues.57 Implant design should be eliminated as a confounding variable when analyzing factors associated with the development of biological complication. However, the lack of standardized and internationally recognized success criteria makes it difficult to compare different studies.58
The treatment modality should result in regeneration of the lost peri-implant tissue. All of the treatment modalities mentioned here have been used by various authors with varying degrees of success, yet there is no standardization for the treatment of peri-implantitis. Surgical treatment in which the lost bone and soft tissue is re-established through bone grafting shows promising results.
The most important step in the avoidance of peri-implantitis is maintenance. Implant patients should visit the dental office every 3 months, where they can be instructed to maintain good oral hygiene, including rinsing with chlorhexidine; using dental floss, gauze strips, yarn, or thicker dental floss or dental tape; and brushing with soft sulcular toothbrushes and powered brushes that are safe to use around the titanium abutment.