You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Hepatitis implies injury to the liver characterized by the presence of inflammatory cells in the tissue of the organ. The name is derived from the ancient Greek word hepar meaning liver and the suffix -itis, meaning inflammation.1
Almost all cases of acute viral hepatitis are caused by one of five viral agents: Hepatitis A virus, Hepatitis B virus (HBV), Hepatitis C virus, the HBV-associated delta agent or Hepatitis D virus, and Hepatitis E virus. Other transfusion-transmitted agents (eg, hepatitis G virus and TT virus) have been identified but do not cause hepatitis.2 All virus types are contagious by blood transmission during active infection.3
HBV infection is a global public health problem, and is endemic in the developing world. Nearly 2 billion people worldwide have been acutely infected by HBV, and there are nearly 300 million people chronically infected with HBV.4 The situation is even more grim in developing nations, including India. Nearly 3% to 4% of the population in India is infected by the virus, and chronic HBV constitutes more than 50% of the chronic hepatitis.5
HBsAg is a serologic hallmark of HBV. Enzyme-linked immunosorbent assay (ELISA) is considered a reliable indicator of detection of the disease. Because as little as 0.0001 mL of infectious blood can transmit the disease,3,6 serological testing is hazardous. In addition, obtaining blood samples for the large-scale detection of HBV is inconvenient because of the extra equipment required. There is also the potential risk of disease transmission through needlestick injuries.5 In addition, the patient population may be reluctant to subject themselves to the invasive procedure for taking blood samples.
The use of saliva as an alternative to blood provides substantial advantages in sampling convenience. It is an easily available diagnostic medium, which can be obtained by noninvasive methods. Literature reports indicate that viral hepatitis markers can be detected in saliva.7 This study aims to establish saliva as a reliable diagnostic tool for detecting HBsAg using ELISA, simplifying the diagnosis of HBV. This can serve as a boon for both dentists and patients as well as in endemic situations.
Materials and Method
The study comprised 70 patients, of whom 35 were known to be HBV seropositive. This group served as the test group. The remaining 35 did not have HBV and served as the control group. The seropositive patients were selected from those admitted to the MM Institute of Medical Sciences and Research, Mullana, India for treatment or were selected from the asymptomatic chronic HBV carriers list available in the blood bank of the MM Institute of Medical Sciences and Research.
The patients’ written consent was then obtained, and approval for the study was obtained from the Ethical Committee of the University. A routine physical examination to assess the vital signs and mental consciousness of each patient was carried out. Clinical signs indicative of HBV infection such as pallor, cyanosis, icterus, pedal edema, and hepatosplenomegaly were noted (Figure 1). A thorough oral examination was then carried out. Unstimulated whole saliva (2 mL) was collected from each patient by the spitting method (ie, the saliva was allowed to accumulate in the mouth of the patients and then they were asked to spit it into a wide-mouthed, plastic container). The samples were then centrifuged for 15 minutes and the supernatant was collected in cryovials and labeled. These samples were then stored at -20ºC until analysis. For serum samples, 2 mL of blood sample was collected from the anticubital vein in the antecubital fossa under complete aseptic conditions. This was centrifuged at 2,000 rpm for 5 minutes and the serum was separated and stored at -20ºC for later determination of HBsAg antigen. All the subjects were assessed for the serum and salivary titres of HBV surface antigen with the help of a HBsAg detection kit (TRANSASIA®, www.transasia.co.in). The findings were then subjected to statistical analysis.
Results
The student t test showed that there was no significant difference between the mean ages of the test and control groups. The mean age was 39.49 ± 14.68 years for the test group and 36.40 ± 3.05 years for the control group, with P = 0.228.
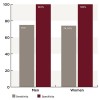
In the test group, the number of women was eight (22.9%); the number of men was 27 (77.1%). The number of women in the control group was 11 (31.4%), while the number of men was 24 (68.6%). The total number of men was 51 (72.9%) and the total number of women was 19 (27.1%).
The sensitivity of using saliva for the detection of HBsAg antigen was found to be 74.29% while the specificity of the study was 100%. The relation between saliva and HBsAg detection was found to be highly significant. The Kappa value came out to be .742, which is highly significant at .001, indicating a very good relation between saliva and HBsAg detection (Table 1).
In addition, it was found that out of eight positive serum samples for women, six cases were positive in saliva and two were negative, with a sensitivity of 75%. Out of 27 positive serum samples for men, 20 were positive in saliva and seven were negative, with a sensitivity of 74.04%. The Kappa value for men was .740 and the Kappa value for women was .776, depicting a highly significant correlation.
Out of 11 negative serum samples for women, all were negative in saliva with a specificity of 100%. Out of 24 negative serum samples for men, all were also negative in saliva, giving the specificity 100% in both men and women (Figure 2).
Discussion
Since the discovery of the Australian antigen better known as HBV surface antigen,8 serological tests have added a new dimension in the early and efficient diagnosis of HBV. HBsAg is considered to be the hallmark of HBV infection. But with these advantages, serum collection and testing carries the disadvantages of being potentially infectious and time-consuming.
HBV surface antigen, or HBsAg, has been reported in feces, urine, bile, semen, tears, and saliva.5,9 The constitutional symptoms of HBV infection include fever, anorexia, nausea, myalgia, jaundice, or icteric hepatitis. These symptoms were reported in only 20% of the positive patients. More than 80% of the patients had subclinical or anicteric hepatitis.
There are two possible explanations for the asymptomatic nature of such patients. First, there could have been perinatal or early childhood transmission of the virus to these patients. Such patients are usually asymptomatic but carry a high risk of chronicity. Second, they could have had subclinical/anicteric hepatitis in the past, and the constitutional symptoms experienced by these patients may have been overlooked.10
In 1976, Peterson and colleagues conducted a study in two Alaskan villages and concluded that saliva and cutaneous exudates containing HBV viruses may play a role in the transmission of HBV.11 Though saliva could be collected by various methods, the most suitable method for saliva collection is the one that permits the collection of adequate amounts of a saliva sample with ease and that contains adequate amounts of quality compound for detection during testing. Collection of whole saliva is easy as it requires minimal armamentarium and training and has more patient compliance. Whole saliva contains a detectable quantity of HBsAg, which is derived from gingival crevicular fluid. Whole saliva is considered a better saliva sample for HBsAg in contrast to stimulated saliva.12,13 Therefore, in the present study, whole saliva was used to detect the presence of HBsAg.
The presence of HBsAg in saliva can be explained by the admixture of the crevicular fluid with saliva. In the present study, the authors chose to detect only HBsAg and not the other HBV antigens because HBsAg is a reliable marker of HBV infection and it is the first antigen to appear after the infection. HBsAg appears in serum as early as 1 to 10 weeks after acute exposure to HBV and even before the prodromal phase of acute HBV. It is also the last antigen to disappear after remission of the infection.2,3 The test is highly economical compared to other serological tests for HBV infection.2 The cost of the test is around just $0.33.
The results of this study conclude that saliva was sensitive to HBsAg in 74.29% seropositive cases. In another study conducted by Wanjari and colleagues in 1998 in Amravati, India, the sensitivity came out to be 76%.14 This is in agreement with the results of the present study because of geographical variance on the Indian subcontinent and the fact that the same standard methodology and regimen was followed.
Broderson and colleagues7 examined 67 patients with liver diseases for the presence of HBV surface antigen in saliva. HBsAg was found in the saliva of 40 out of 48 seropositive patients with a sensitivity value of almost 83%. In 1997, Clemmons and colleagues15 conducted a study in which the results indicated a sensitivity of 87%. The high sensitivity in these studies was most likely a result of the fact that they used the dipstick method for the detection of HBsAg in saliva. The sensitivity could have been higher in this study if the negative salivary samples of the seropositive patients were subjected to repeat testing, which was difficult and time-consuming because of a lack of patient compliance.
The probable reason for the absence of HBsAg in the saliva of some patients could be that the concentration of HBsAg in saliva is lower than in blood, or the patient could be a chronic carrier of the HBV infection. This low concentration of the antigen in saliva might limit its detection even with the ELISA method. A wide variability in the frequency of detection may be a result of the variation in the mode of collection and handling of saliva. Ben-Aryeh and Ben-Porath16 established a sensitivity of 90% and a specificity of 100% in assessing salivary HBsAg using ELISA. Thieme and colleagues13 established a sensitivity and specificity of 100% for the detection of HBsAg in saliva and put forth a conclusion that oral sampling represented a safer and more convenient procedure than blood sampling and that it had wide application in both patient and outbreak management.
Conclusion
The present study shows a great deal of success in achieving the mentioned objectives. It also shows that saliva is an infectious agent, which has serious implications for dental personnel. The key to accuracy seems to lie in the method of oral fluid collection and in precision of the test assay.
The testing used in this study has been shown to be simple and economical. It is noninvasive, which ensures better patient compliance. It also appears to be a safe and fairly accurate method for the detection of HBsAg. Saliva can be used as a medium for the detection of HBsAg and is a promising tool for epidemiological and diagnostic studies.
References
1. Hurlen B, Jonsen J, Netland A, Osnes S. Salivary HBsAg in hepatitis B infection. Acta Odontol Scand. 1980;38(1):51-55.
2. Porter S, Scully C, Samaranayake L. Viral hepatitis: Current concepts for dental practice. Oral Surg Oral Med Oral Path. 1994;78(6):682-695.
3. Koff RS, Isselbacher KJ. Changing concepts in the epidemiology of viral hepatitis. N Engl J Med. 1968;278(25):1371-1380.
4. World Health Organization (WHO). Hepatitis B fact sheet N°204. http://www.who.int/mediacentre/factsheets/fs204/en/index.html. Accessed January 20, 2012.
5. Hersh T, Melnick JL, Goyal RK, Hollinger FB. Nonparenteral transmission of viral hepatitis type B (Australia-antigen-associated serum hepatitis). N Engl J Med. 1971;285(24):1363-1364.
6. Feinman SV, Berris B, Guha A, et al. DNA: DNA hybridization method for the diagnosis of hepatitis B infection. J Virol Methods. 1984;8(3):199-206.
7. Broderson M, Stegmann S, Klein KH, et al. Letter: Salivary HBAg detected by radioimmunoassay. Lancet. 1974;1(7859):675-676.
8. Braunwald E, Fauci AS, Kasper DL, et al. Harrison’s Principles of Internal Medicine. Vol 2. 15th ed. McGraw Hill; 2001:172.
9. Gitnick LG, Goldberg LS, Koretz R, Walsh JH. The liver and the antigens of Hepatitis B. Ann Intern Med. 1976;85(4):488-496.
10. Reingold AL, Kane MA, Hightower AW. Failure of gloves and other protective devices to prevent transmission of Hepatitis B virus to oral surgeons. JAMA. 1988;259(17):2558-2560.
11. Petersen NJ, Barrett DH, Bond WW, et al. Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages. Appl Environ Microbiol. 1976;32(4):572-574.
12. Tanno G, Fay O, Roncoroni M. Virus-B Hepatitis in saliva. Lancet. 1972;2(7781):822-823.
13. Thieme T, Yoshihara P, Piacentini S, Beller M. Clinical evaluation of oral fluid samples for diagnosis of viral hepatitis. J Clin Microbiol. 1992;30(5):1076-1079.
14. Wanjari PV, Wanjari S, Potode A. Hepatitis-B and Dentistry: A Review. J Indian Dent Assoc. 1998;69:127-132.
15. Clemmons RM, Stewart C, Davis G, et al. Development of a prototype, rapid saliva test for hepatitis B surface antigen (HBSAg) utilizing a “dipstick” method. Ann N Y Acad Sci. 1993;694:272-273.
16. Ben-Aryeh H, Ben-Porath E. The relationship between antigenemia and excretion of Hepatitis B surface antigen in human whole saliva and in gingival crevicular fluid. Arch Oral Biol. 1985:30(1):97-99.
About the Authors
Gagandeep Arora, MDS
Assistant Professor
Department of Oral Medicine and Radiology
MNDAV Dental College and Hospital
Tatul, Solan, Himachal Pradesh, India
Soheyl Sheikh, MDS
Professor and Head
Department of Oral Medicine and Radiology
MM College of Dental Sciences and Research
Mullana, Ambala, Haryana, India
Shambulingappa Pallagatti, MDS
Professor
Department of Oral Medicine and Radiology
MM College of Dental Sciences and Research
Mullana, Ambala, Haryana, India
Balwinder Singh, BDS
Postgraduate Student
Department of Oral Medicine and Radiology
MM College of Dental Sciences and Research
Mullana, Ambala, Haryana, India
Varsha A. Singh, MD Professor
Department of Microbiology
MM Institute of Medical Sciences and Research
Mullana, Ambala, Haryana, India
Ravinder Singh, MDS
Senior Lecturer
Department of Oral Medicine and Radiology
MM College of Dental Sciences and Research
Mullana, Ambala, Haryana, India