You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
With the evolution of dental implant therapy, the treatment of extraction sockets has progressed from a simple matter of wound healing to what is often times a complex surgical procedure aimed at minimizing bone resorption. The consequences of physiologic wound healing from extractions often include both vertical and, more prominently, lateral reductions of the local alveolar process. The healing of extraction sockets is accompanied by marked ridge resorption within the first 3 to 4 months.1 Schropp demonstrated approximately 50% horizontal bone loss 12 months after extractions.2 Nevins et al demonstrated that when extraction of teeth with prominent roots are augmented with grafts, when compared to ungrafted sites the grafted sites facilitated favorable implant placement. Significantly fewer of the grafted sites required additional grafting procedures at the time of implant placement.3
Although many "ridge augmentation" techniques exist, it is certainly more efficacious for both surgeons and patients to prevent bone modeling that results in physiologic resorption and necessitates more involved modalities. The term "socket preservation" usually refers to the placement of various bone replacement grafts that are often covered with a barrier membrane. The bone graft materials are used to maintain space and serve as an osteoconductive scaffold to support passive osteogenesis within the "pores" both between and within the graft particulate. Iasella et al demonstrated significantly greater 3-dimensional (3-D) ridge preservation for extraction sockets augmented with allograft bone and collagen membranes compared to ungrafted controls.4 Araújo et al demonstrated in the canine model that the pores of tricalcium phosphate particulate can be invaded by erythrocytes, and that later these pores would become the locus of new bone formation. These same authors also noted that a degree of delayed healing and minimal bone formation occurred between the second and fourth weeks of recovery. These authors speculated that the β-TCP (beta-tricalcium phosphate) graft may have retarded bone formation.5 In a review article, Darby et al concluded that ridge preservation is an effective procedure in limiting both horizontal and vertical ridge alterations in post-extraction sites, and that there is no technique superior to another.6
The barrier membrane provides a soft-tissue exclusionary function, blocking the ingrowth of epithelial and fibroblastic cells and favoring the repopulation of osteoblast cells for bone replacement of the graft material. Investigators such as Carmagnola et al reported excellent clinical results when particulate xenograft coverage, without soft-tissue closure, was achieved when a collagen membrane was adapted over the bone graft and beneath the mucoperiosteal flap.7 The majority of these procedures provide 3-D bone volume, facilitating prosthetically driven implant placement. The caveat of these treatments is that osseointegration occurs to support long-term tooth replacement that may involve permanent inclusion of graft material.
At the inception of dental implantology, the phenomenon of osseointegration was investigated through histologic animal studies. Titanium implants were inserted into healed alveolar ridges, composed of "native" bone. Most long-term (over 10 to 15 years) studies followed these types of clinical situations. It would seem logical that ideal clinical situations would support the possibility of implant placement into sites composed of native bone, excluding bone graft materials occupying spaces of potential bony trabeculae. The challenge that still exists today, when teeth require extraction and site preparation is chosen to facilitate future implant placement, is to regenerate de novo bone and maintain osseous morphology favorable for restoratively driven implant placement.
Recombinant technology has given surgeons the ability to stimulate wound healing and cellular differentiation, leading to tissue regeneration. The stimulatory properties of these peptides require vehicles for sustained delivery. Some of these growth factors are commercially available in combined packaging, with bone grafting particulate as the delivery vehicle. These materials possess some degree of osteoconductivity and occupy a physical space, preventing maximum osseous fill of the desired space. Some of these growth factors are not specific for osteoblastic differentiation.
One of the few commercially available recombinant growth factors proven to be selectively osteoinductive is recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) (INFUSE® Bone Graft, Medtronic, Inc. [FDA PMA submission number for INFUSE OMF indication is P050053.]). rhBMP2 is a differentiation factor that changes the phenotype of precursor cells (mesenchymal stem cells) into osteoblasts and chondroblasts. The standard dose of the protein is 1.50 mg per 1 cc of the solution. The lyophilized rhBMP-2 is combined with sterile water chairside. Once the solution is mixed, it is uniformly dispensed onto an absorbable collagen sponge (ACS). Following a minimal saturation time of 15 minutes, this collagen sponge can be cut into various size strips to be delivered to the site of desired regeneration. The release of the growth factor is sustained over an approximately 2-week time period. Because the ACS is not treated by crosslinking to delay degradation, it is resorbed quickly, leaving no remnants of graft material at the placement site. This facilitates a maximum potential for bone-fill of the grafted defect. The stimulatory regenerative and vascular invasion effects of rhBMP-2 also accelerates bone formation. This is significant because it can shorten the overall treatment time for patients.
Purpose
The purpose of this article is to present a case series of consecutively treated patients (Table 1) who at the time of molar tooth extractions received grafting of the alveolus with the rhBMP-2/ACS material alone. This retrospective analysis fully complies with the Helsinki Accords and Ethical Guideline for Clinical Research. All patients included in this case series signed written consent forms that explained the nature of the procedure undertaken, stating that they agreed to undergo the prescribed therapy. These patients were also informed that the material used for augmentation was a recently FDA-approved material that was indicated for grafting of extraction sockets.
All grafted sites received dental implants within a 3- to 6-month time period following extraction. Surgical procedures were not altered in terms of underpreparation, bone condensing, or additional grafting at the time of implant placement, with the exception of the initiation of osteotomies with a small (2.7-mm outer and 2-mm inner diameter) trephine. The cores harvested were submitted for qualitative histologic evaluation to confirm the presence of vital bone. All implants subjectively achieved primary mechanical stability and were restored 3 to 8 months after placement.
Case 1
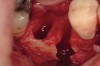
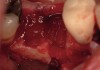
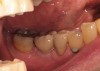
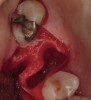
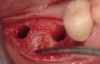
The patient was a 71-year-old man with significant caries and subsequent bone loss associated with tooth No. 30. The septal bone was lost, with the exception of the coronal aspect, resulting in a "bridge of bone" connecting the buccal and lingual cortices of the site (Figure 1). After reflection of the full-thickness buccal and lingual flaps, extraction, and manual and ultrasonic debridement of the socket to remove all visible soft-tissue remnants, the defect was obturated with the rhBMP-2/ACS material (Figure 2).
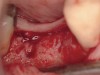
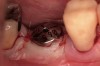
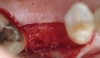
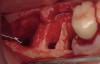
Fifteen weeks after the first procedure, the site was reopened to perform implant placement. Flap reflection revealed excellent and complete bone reformation (Figure 3). The implant osteotomy preparation was initialized with the harvest of an approximate 5-mm trephine core. The trephine had an internal diameter of 2 mm and an outer diameter of 2.7 mm. The completion of implant placement was performed according to the manufacturer's guidelines, resulting in the delivery of a 5-mm x 11-mm implant with primary stability. Because of excellent subjective stability, a transmucosal healing was chosen, with placement of a healing cap and a nonsubmerged closure (Figure 4). Qualitative histology demonstrated lamellar bone without evidence of the ACS carrier. Restorative therapy commenced approximately 4 months after implant placement. Delivery of the definitive prosthesis, consisting of a gold custom abutment and cement-retained crown, occurred at 5 months following implant placement surgery and 8.5 months after extraction and augmentation (Figure 5).
Case 2
The second patient was a 75-year-old woman who presented with a chronic infection associated with a fracture of the distobuccal root of tooth No. 3. Following flap reflection, complete buccal bone loss was associated with the root fracture. Tooth No. 3 was extracted, with all remaining bony walls of the extraction socket being preserved (Figure 6). Debridement was followed by obturation of the defect with the rhBMP-2/ACS (Figure 7). A subepithelial, connective tissue pedicle graft was rotated to provide partial coverage of the grafting material. The graft was then closed with a monofilament polytetrafluoroethylene (PTFE) suture.
Approximately 18 weeks following extraction and grafting, full-thickness flaps were reflected, revealing complete osseous regeneration of the original defect (Figure 8). The osteotomy was initiated with the same trephine bur to harvest a core of representative bone present at the site of implant insertion. Implant placement proceeded without alteration from the manufacturer's protocol by inserting a 4.8-mm x 8-mm fixture with primary, tactile, stability, and transmucosal healing properties. At about 8 months postplacement, the implant was restored with a custom abutment and cement-retained crown (Figure 9).
Case 3
The patient was a 66-year-old man who required removal of the three mandibular right molars due to rampant caries and attachment loss. Following flap reflection and extractions, the sockets were debrided with both ultrasonic and manual instrumentation (Figure 10). The sockets of the first and second molars were augmented with rhBMP-2/ACS. The site of the third molar was obturated with a noncrosslinked, collagen plug for hemostatic purposes only.
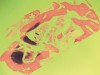
The restorative treatment plan encompassed tooth replacement in the first and second molar positions only, negating the need for the patient to incur the greater expense of augmenting the third molar site. Primary closure was achieved with a monofilament PTFE suture. Approximately 6 months after the extractions and augmentation procedure, the patient returned for implant placement surgery. Surgical reopening revealed excellent visual regeneration and ridge preservation (Figure 11). The site of the tooth No. 31 osteotomy was chosen for biopsy harvesting, because this is where the most severe bone loss existed at the time of extraction, and this site would be most representative of new bone formation, as opposed to possibly harvesting pre-existing native bone. This trephine core qualitatively revealed what was diagnosed by the histopathologist as "normal bone" without any foreign body or inflammatory responses being evident (Figure 12). Serving as a historic control, Trombelli et al reported on histomorphometric measurements of various tissues present at different time intervals. These authors describe great variability in human trephine cores taken from extraction sites. In relation to the present case series, Trombelli et al described the presence of a provisional matrix and woven bone dominating what they described as late-phase healing taken at 12 to 24 weeks after extractions. Although tissue modeling was described as fast, the authors found the remodeling of the newly formed bone to be what they called "seemingly slow." The trephine core presented in this particular case demonstrated this type of healing, as described by Trombelli et al.8 A high degree of woven bone as well as a cell and fiber-abundant provisional matrix was present.
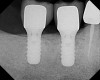
Two 4.8-mm x 10-mm implants were placed using standard protocol. These implants achieved primary stabilization and facilitated transmucosal healing. At just under 20 weeks, the two implants were restored with two individual cement-retained crowns (Figure 13).
Findings
All of the consecutively treated patients underwent extraction of molar teeth, simultaneous bone augmentation with rhBMP-2/ACS, and implant placement approximately 4 to 6 months after the first procedure. There were several key findings. First, all of the augmented sites facilitated restoratively driven implant placement that was not possible at the time of extraction because of bony insufficiency. Second, all implants subjectively achieved primary stability. No mobility or rotation of the implants occurred upon tightening of the healing abutments. Third, no additional bone augmentation was necessary at the time of implant placement in any of these cases.
Implant placement resulted in circumferential bony coverage of the implant surfaces either to the collar of the implants, or to the rough–smooth titanium border, depending on the implant type used in each individual situation. The most common adverse event or morbidity was mild to moderate postoperative edema that was noted intra- and extraorally. This reaction to oral grafting with rhBMP-2/ACS has also been reported by Boyne et al.9 These same investigators detected antibody production to rhBMP-2 in a small percentage (12%) of patients treated with a therapeutic dosage of 1.50 mg/mL. This was a transient finding that did not affect treatment outcomes or require further treatment.
It is also worth mentioning that although the retrieved trephine cores revealed qualitative evidence of healthy bone, without evidence of persisting graft material or adverse cellular reactions, histomorphometry was not performed. The percentages of woven bone, lamellar bone, and soft tissue within the cores were not evaluated. The purpose of the histologic component of this report is to demonstrate the presence of viable native bone, without undesired inflammatory processes or residual graft materials, in the locations of implant insertions.
Most importantly, from a clinical perspective, all consecutively placed implants in this case series achieved osseointegration and were functionally loaded in a standard period of time (3 to 8 months). Delayed loading due to poor subjective bone quality or suboptimal implant stability was not found with any of the implants in this case series.
Discussion
Ridge preservation is a frequently investigated subject. Numerous combinations of bone replacement grafts, barrier membranes, and the addition of various growth factors have been evaluated. The primary goal of these procedures is to preserve and/or regenerate alveolar bone associated with extraction sockets and prevent the anticipated, physiologic bone resorption that follows tooth loss. Araújo et al found that when canine extraction sockets are augmented with Bio-Oss Collagen® (Osteohealth, www.osteohealth.com), some of the expected dimensional alterations could be offset. The collagen portion of the graft was readily eliminated, whereas the xenograft portion of the graft persisted, although bone formation occurred on the surface of the graft particles.10 The presence of newly formed bone onto the nonresorbable graft surface demonstrates the passive process of osteoconduction. Graft particles are placed into the site and the repopulation of the bone-forming cells occurs over time and is dependent on the individual defect's and patient's ability to heal and regenerate lost or damaged tissue. This process lacks a stimulatory cellular component.
According to Lane et al,11 the tissue engineering model is composed of three elements. First, each site of regeneration requires that cells be capable of differentiating into the desired cell line needed to regenerate the desired lost tissue. Second, a signaling molecule is required to provide chemotactic, morphogenic, and differentiation messages to these cells. Third, the signaling molecule and migrating cells require a scaffold or matrix to provide the physical space necessary to carry the message to the site and facilitate cellular migration.
The fate of implants placed under functional, occlusal load in sites augmented with any bone graft is a primary concern for clinicians and patients. The possibility of placing implants is the first step in a multistep process of tooth replacement. Initial stability, followed by secondary stability or osseointegration, is the specialty of the restorative dentist. Treatment is considered a failure if these implants do not function in a healthy state, under normal occlusal conditions. In an animal model, Jovanovic et al demonstrated that machined titanium implants can function for 12 months after placement in sites of experimental defects augmented with rhBMP-2/ACS 3 months prior to placement. Not only were these implants successfully loaded for 1 year, but the authors also noted that the bone-to-implant contact (BIC) for fixtures inserted into rhBMP-2 grafted sites was comparable to implants placed into native bone.12 In a pilot study conducted in humans, Cochran et al evaluated a subtherapeutic dose of rhBMP-2 (0.43 mg/cc) in extraction sockets or ridge augmentations. The 3-year results demonstrated safety and long-term efficacy of this growth factor for site development facilitating implant placement.13 In a randomized controlled study, Fiorellini et al evaluated rhBMP-2/ACS delivered at 1.50 mg/cc, 0.75 mg/cc, ACS alone, and ungrafted controls in the treatment of maxillary extraction sockets with buccal wall defects.14 Significant ridge height was preserved, and bone width was regenerated when the sites were augmented with rhBMP-2/ACS. Ungrafted and ACS-only grafted sites demonstrated little bone regeneration. The sites grafted with the commercially available dosage of 1.50 mg/cc of rhBMP-2 outperformed the sockets grafted with the subtherapeutic dose of 0.75 mg/cc in terms of dimensional bone maintenance and regeneration. These investigators evaluated anterior sites for socket augmentation and preservation.