You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The most effective approach to maintain gingival and periodontal health is to regularly remove the accumulating bacterial biofilm. Dental biofilm's extended presence on the tooth surface, adjacent to the gingiva, can lead to gingivitis and subsequently periodontitis. Dental plaque biofilm can be mechanically removed by toothbrushing. However, there is a higher risk for developing periodontal diseases in interproximal spaces, when the microbial plaque is not totally removed. Interproximal spaces are naturally difficult to reach for proper mechanical plaque removal. Dental anatomy and position in the dental arch vary among individuals, making it difficult to effectively remove plaque from all surfaces. Manual dexterity and motivation for maintaining proper oral hygiene are also important factors to be considered. Use of interproximal plaque removal devices, such as dental floss and interdental brushes, are considered to be an integral part of proper oral hygiene. There have been numerous clinical studies demonstrating that the combination of using a toothbrush and interproximal cleaning devices improves the reduction in plaque biofilm accumulation and gingivitis.1,2
Various types of interproximal cleaning devices have been developed and released into the marketplace with the goal of improving patient compliance with interdental plaque control. Since their release, interdental brushes have gained popularity, due to their ease of use. The rubber bristle interdental cleaner is one of the many types of interdental cleaning devices on the market; however, its design is unique when compared to other similar devices. Its design uses gentle rubber bristles (small ridges), as compared to devices with regular nylon bristles, which can potentially cause gingival irritation and trauma.3,4 The longer handle in combination with a curved design offers advantages for effectively reaching interdental spaces that may otherwise be inaccessible, such as between premolars and molars.
The purpose of this study was to evaluate the efficacy of a curved rubber bristle interdental cleaner (cRBIC), as compared to an ADA-accepted leading brand dental floss (Floss), and examine how ease-of-use can promote the establishment of a hygienic routine of cleaning interproximal spaces in patients with gingivitis.
Methods
Study design
This parallel design, examiner-masked, randomized controlled clinical study took place at a single center from December 2016 to April 2017. Fifty evaluable patients diagnosed with gingivitis only were recruited from the General and Oral Health Center at the University of North Carolina (UNC), Chapel Hill, campus and were randomized with 2% attrition. The study was conducted according to the International Conference of Harmonization Guideline for Good Clinical Practices. All study materials and protocols were reviewed and approved by the University of North Carolina Institutional Review Board (IRB) prior to enrollment of the study participants. No changes occurred in the trial design after commencement of the study and the study was registered at clinicaltrials.gov.
Study population
Male and female volunteers aged 18-70 years, considered to be in good general health, who could read; understand; agree to provide consent; and who were able and willing to follow study procedures and instructions were recruited to participate. Participants had no or little experience with interproximal cleaning devices, such as dental floss or interdental brushes. Each participant needed a minimum of 20 "scorable" teeth, excluding 3rd molars, with bleeding on probing (BOP) sites more than or equal to 10% and less than or equal to 50% of the mouth. Periodontal pocket depths needed to be less than or equal to 4 mm. Participants also needed to be non-smokers and have a minimum of 12 qualifying, interproximal units (3 per quadrant), with closed contacts without crown or restorations. Table I summarizes the demographics and clinical measurements obtained at enrollment.
Study products
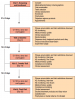
Treatment products were dispensed to participants according to their randomization assignment. Participants received either the curved rubber bristle interdental cleaner (cRBIC) (GUM® Soft-Picks® Advanced; Sunstar Americas, Inc.; Chicago, IL, USA) as the test product; or dental floss (Floss) (Oral-B® Pro-HealthTM Glide® Original; Procter & Gamble; Cincinnati, OH, USA) as the control product. The test product features soft, flexible tapered rubber bristles on a curved handle, allowing for easy access any area of the mouth (Figure 1). The dental floss control product received the American Dental Association (ADA) seal of acceptance, and is lightly coated with natural wax for improved grip while its silky-smooth texture slides easily between teeth.
Study procedures
The study flow is illustrated in Figure 2. At the screening appointment (visit 1), informed consent was obtained from potential participants followed by a medical/dental history and oral examination performed by the study coordinator. Twelve interproximal units were selected and qualified (3 units per quadrant)to collect the Modified Quigley-Hein Plaque Index (MQH-PI)5-8 and modification of the Löe and Silness Gingival Index9 (GI). For the purposes of this study, grading included only interdental spaces. The categories were as follows: 0 = normal gingiva (pink, firm, stippled); 1 = mild inflammation: slight change in color, slight edema, no bleeding on probing; 2 = moderate inflammation: glazing, redness, edema, bleeding on probing; 3 = severe inflammation: marked redness and edema, ulceration, tendency to spontaneous bleeding. Full-mouth periodontal probing depth (PPD) and bleeding on probing (BOP)10 were assessed at 6 sites per tooth.
Participants who met the eligibility criteria were enrolled and were provided with a dental prophylaxis, consisting of scaling and polishing. Enrolled participants received the same oral hygiene care products (Oral-B® Indicator manual toothbrush and Crest® Cool Mint Gel dentifrice; Procter & Gamble; Cincinnati, OH, USA) and instructed to use the products twice daily throughout the study period. Visit 2 was scheduled 21 days after the screening enrollment appointment. Participants were instructed to perform toothbrushing 12-18 hours before their scheduled appointment. They were also instructed to refrain from chewing gum and other hard crunchy foods during 3 to 6 hours before the study appointment.
Clinical assessments (MQH-PI, GI, PPD and BOP) were carried out in the previously designated sites by calibrated examiners at visit 2. Examiners were blinded to which group each participant was assigned, until conclusion of the efficacy evaluations. Participants were randomly assigned to either the test group (cRBIC) or the control group (Floss) and instructed to use the assigned device once a day (afternoon or evening), at approximately the same time. Appropriate written instructions for the assigned product were provided, along with a detailed review of product use by a member of the research staff, at the time the assigned product was dispensed. Participants demonstrated back their understanding of the interdental cleaning instructions during the supervised session. Participants were also provided an at-home user experience diary, as well as a compliance diary. The diary indicated the level of motivation to interdental cleaning and satisfaction to the assigned product.
Adverse events were monitored by interview; a health history update and oral examination took place at each visit following enrollment. Research staff performed a compliance check with a verbal interview and review of the diary at visits 3 and 4. Lack of compliance was recorded as follows: failure to follow brushing instructions; failure to complete study diary; failure to follow plaque accumulation instruction; prohibited medication usage; prohibited oral care product usage; and failure to return product. Clinical assessments were performed at visit 3 (MQH-PI, GI only) and visit 4 (all endpoints).
Power calculation and statistical analysis
The sample size was determined based on calculations described by Noordzi et al. for randomized controlled trials comparing two groups of equal size.1 The significance level alpha was set at 0.05. With a minimum of 22 participants per treatment group, the power is at least 90% to detect a difference of 0.25 in interproximal plaque indices, referenced in the results of a similar clinical study by Jackson et al.12
The primary analysis was performed on all randomized participants at baseline, visit 3, and visit 4 (efficacy evaluation; modified intent to treat, MITT). Participants with a compliance level of 75% were grouped according to the randomized treatment assignment. The analysis of safety included all randomized participants who were exposed to treatment. All variables were summarized by descriptive statistics and analyses were conducted using Minitab® 18 (Minitab Inc.; PA, USA). Differences between the two treatment groups' continuous demographic characteristics (e.g., age) were analyzed using Student's t-test. Differences between the groups' categorical characteristics (e.g., sex, race, ethnicity) were analyzed using Chi-Squared test.
Differences for clinical endpoints (changes of measurements from baseline to 2 or 4 weeks) were tested with an analysis of covariance model (ANCOVA) adjusted by baseline data followed by post-hoc Tukey Simultaneous test. Superiority for the continuous effectiveness endpoints at each time point was tested with Mann-Whitney test. Comparisons between baseline and 2 or 4 weeks in clinical measurements in each group were performed by Wilcoxon signed-rank test. Qualitative data of the diary were tested with Student's t-test. The probability level of statistical significance was set to p<0.05.
Results
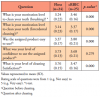
A total of 53 patients were assessed for eligibility, and 50 eligible participants were randomized in nearly equal proportions to either the test group (n=26) or the control group (n=24). One participant of the test group was lost to follow up after 2 weeks. Participant demographics in the two groups was shown to be similar (Table I). There were no statistically significant differences between the control group (Floss) and test group (cRBIC) in regard to measurements of MQH-PI, GI, PPD and BOP at enrollment and baseline (Table II). Measurements of GI and BOP were significantly reduced from enrollment to baseline due to the oral prophylaxis provided at enrollment. There were no changes observed in MQH-PI and PPD between enrollment and baseline. No statistically significant differences were observed in the mean change between the two groups in any clinical measurements from baseline to 2 weeks. A statistically significant difference was detected in the mean change of PPD at 4 weeks between the two groups (test: -0.16±0.21, control: 0.00±0.23, p<0.05), while there was no significant difference in any other clinical parameters (Table III).
Comparing to baseline, PPD was improved significantly in both groups at 2 weeks, and the improvement from baseline was still observed at 4 weeks in the test group (p<0.01). BOP was shown to improve from baseline to 2 weeks in both groups, but these changes were not statistically significant. Between 2 weeks and 4 weeks, BOP returned to a level worse than baseline in both groups; however, a statistically significant difference (p<0.01) was found only in the control group (Table III). There were no statistically significant differences between baseline and 2 or 4 weeks in MQH-PI and GI measurements.
Qualitative information of the diary, as well as clinical endpoints, were analyzed. The cRBIC (test) group demonstrated better compliance levels (%) than the Floss (control) group during the study period followed by the short learning curve (test: 97.4±3.8, control: 94.8±5.6, p<0.05) (Table IV). Mean scores of "ease-of-use" of the test group was significantly greater than that of the control group (p<0.01). The control group indicated better "satisfaction" than test group (p<0.01). Motivations for oral hygiene (brushing and interdental cleaning) were higher in the test group than the control group during the study period (p<0.01). No adverse events were reported during the trial.
Discussion
Interdental cleaning has been a critical aspect of overall effective plaque control and prevention of periodontal diseases, and many publications have addressed this topic previously.13 Recently, a meta-analysis was conducted to evaluate the efficacy of oral hygiene aids.14 Kotsakis et al concluded that there is lack of strong evidence to support one method over another and recommended that practitioners customize oral hygiene methods to meet the needs of the individual. Customized oral hygiene instructions with alternative approaches to meet the needs and preferences of the patient still prevails in practice.
The use of a curved-design rubber bristle interdental cleaner (cRBIC) in this study demonstrated positive outcomes in regard to probing depth reduction and ease-of-use, as compared to dental floss. However, there were no clinically significant improvements in the parameters obtained between baseline and the end of the study in either the control or test groups. The lack of positive change parameters in either group is believed to be due to the professional prophylaxis provided to all participants 3 weeks prior to obtaining the baseline measurements. The oral health effects of the thorough prophylaxis performed by dental hygienists quite likely lasted longer than 3 weeks, thus the GI and BOP at baseline were lower than those at enrollment. However, there were no differences in interproximal MQH-PI score and in PPD between the enrollment and baseline measurements. This finding may indicate that while the plaque accumulation was possibly improved by the prophylaxis and oral hygiene instruction at the first visit, the effect may have been gradually lost during the 3-week washout period. Improvements on BOP and GI may be due to the thorough removal of plaque and calculus at enrollment, with the effect lasting 2 weeks following the study initiation. A minor but significant improvement of PPD from baseline was detected in the test group at 4 weeks. Between 2 and 4 weeks, BOP clearly trended towards the level before professional prophylaxis, and was faster in the control group than the test group (p<0.01) indicating that using a cRBIC may help the effects of professional prophylaxis to last longer than using dental floss.
Rubber interdental cleaning devices, including the cRBIC, have been compared to other interdental cleaning devices in three other clinical studies. Hennequin-Hoenderos et al2 evaluated cRBIC to an interdental brush in participants with experimental gingivitis and found that using a cRBIC produced similar results to that of the interdental brush in plaque reduction. However, gingival bleeding at accessible interdental sites was reduced, when compared to the interdental brush at 4 weeks after the resumption of oral hygiene practices.2 Yost et al15 showed that the effectiveness of RBIC (straight handle) was equivalent to floss in reducing plaque scores and gingivitis levels. The efficacy of the interdental brush was also shown to be superior to other interdental cleaning devices based on the clinical results of Yost et al.15 Another study by Abouassi et al16 comparing a straight handle rubber interdental bristle to an interdental brush demonstrated similar clinical efficacy of the two devices. Plaque accumulation was not improved after either device was used for 4 weeks while bleeding was significantly reduced during the study period by both devices. A professional oral prophylaxis was also provided to all participants prior to the start of both the Yost et al15 and Abouassi et al studies.16 Since the target populations (experimental or spontaneous gingivitis patients); study designs (cross-over or parallel); and study conditions (timing of a professional prophylaxis) are not the same across these studies, some discrepancies in conclusions should be expected. However, when viewing the study results overall, it can be concluded that any type of interdental dental cleaning might be effective on gingival bleeding reduction in varying degrees and may be due to the fact that BOP is a reliable clinical parameter for diagnosing gingivitis.17
The efficacy of interdental cleaning is largely affected by the acceptability of the method by the patient and the degree of compliance to the technique. In the Hennequin-Hoenderos2 study, participants were given a period of time to become familiar with an assigned device. The familiarizing period may have reduced an effect of participant acceptability to an assigned device on the clinical outcome. The results of other studies,1,2 including the current study, may have reflected the effectiveness of the device itself and the participant's acceptance of the assigned device more than Hennequin-Hoenderos2 due to the lack of a familiarizing period. Participants assigned to the cRBIC group showed an increase in the average score for the question on "ease-of-use" in the diary, gradually, during the first week of the study period (data not shown). This finding may indicate that the participants needed a learning period to become familiar with and to accept the new interdental cleaning method.
This study assessed patient acceptability to the assigned method through the use of a diary. The cRBIC group demonstrated significantly higher scores in assessment of motivation for interdental cleaning or brushing, as compared to the Floss group. Perhaps it was the design aspect of the cRBIC of only requiring the use of one hand and its unique long, curved handle, as compared to the straight design, that may have made it more user-friendly. Patients may be unable to use dental floss properly, due to challenges in maneuvering the floss interdentally, with both fingers in the mouth. Participants in the cRBIC group in this study gave a higher score in the handling property (ease-of-use) category than that of the Floss group. Ease-of-use of the cRBIC device may have affected the participants' motivation for interdental cleaning on a daily basis, ultimately resulting in better compliance. While the Floss group gave higher scores in satisfaction than the cRBIC group, satisfaction with the device did not seem to promote increased motivation for interdental cleaning. This finding can be interpreted to mean that while participants appreciated the performance of floss; this was not enough to motivate them towards the habit of interdental cleaning.
Patient acceptance of the interdental cleaning tools, through questionnaires at the end of the study period, was used in the Abouassi et al16 comparing rubber interdental brushes to a control interdental brush. Rubber interdental bristles were given high scores for manageability of the device, less pain during usage, comfort of brushing, and willingness to buy the product, as compared to the control brush. Considering these findings, rubber bristle interdental cleaning devices, including the test device, can be used as an alternative interdental cleaning aid as an alternative to floss products or interdental brushes due to the high level of patient acceptance. Findings from this study suggest that using a cRBIC may be advantageous in reducing and maintaining lower levels of gingivitis and may promote a motivation to daily interdental cleaning due to its high level of patient acceptance. Future studies should be of longer duration (minimum of 3 months) to monitor the efficacy of the clinical outcomes over a maintenance period.
Conclusions
A curved rubber bristle interdental cleaner was similar to an ADA seal of acceptance dental floss product in clinical performance; however, the PPD reduction at 4 weeks was greater in the cRBIC group. Measurements of BOP in both groups trended toward levels recorded prior to professional oral prophylaxis; however, use of the cRBIC appeared to help maintain the effects of the prophylaxis longer. Ease of use of the cRBIC may have affected the participants' motivation for interdental cleaning, resulting in better compliance, as compared to dental floss.
Acknowledgments
Dr. Steven Offenbacher passed away unexpectedly in August 2018. A passionate scientist, educator and mentor, he was also humble, with great sense of humor and a warm heart. Dr. Offenbacher was highly respected worldwide for his research, especially in the area of periodontal medicine. He introduced pioneering research on periodontal disease and pregnancy outcomes. His research on the understanding of periodontal disease and cardiovascular diseases made a high impact in both medicine and dentistry. Dr. Offenbacher inspired, mentored and influenced scores of students and professionals over the span of his career. The authors want to acknowledge his significant contributions and mentorship for this research project.
About the Authors
Antonio J. Moretti, DDS, MS is an associate professor, Division of Comprehensive Oral Health, Periodontolgy, Adams School of Dentistry, The University of North Carolina, Chapel Hill, NC.
Shaoping Zhang, DDS, MS, PhD is an assistant professor, Department of Periodontics, University of Iowa College of Dentistry, Iowa City, IA.
Sherrill T. Phillips, RDH, BS is a dental research project manager, Adams School of Dentistry, The University of North Carolina, Chapel Hill, NC.
Kristy M. Williams, RDH is a project specialist, Syneos Health, Morrisville, NC.
Kevin L. Moss is an applications specialist, Division of Comprehensive Oral Health, Adams School of Dentistry, The University of North Carolina, Chapel Hill, NC.
Steven Offenbacher, DDS, MMSc, PhD was the former chair, Department of Periodontology, Adams School of Dentistry, The University of North Carolina, Chapel Hill, NC.
Corresponding author: Antonio J. Moretti, DDS, MS; Antonio_Moretti@unc.edu
References
1. Sälzer S, Slot DE, Van der Weijden FA, Dörfer CE. Efficacy of inter-dental mechanical plaque control in managing gingivitis--a meta-review. J Clin Periodontol. 2015 Apr;42 Suppl 16:S92-105.
2. Worthington HV, MacDonald L, Poklepovic Pericic T, et al. Home use of interdental cleaning devices, in addition to toothbrushing, for preventing and controlling periodontal diseases and dental caries. Cochrane Database Syst Rev. 2019 Apr 10;4:CD012018.
3. Hennequin-Hoenderdos NL, van der Sluijs E, van der Weijden GA, Slot DE. Efficacy of a rubber bristles interdental cleaner compared to an interdental brush on dental plaque, gingival bleeding and gingival abrasion: A randomized clinical trial. Int J Dent Hyg. 2018 Aug;16(3):380-8.
4. Anneroth G, Poppelman A. Histological evaluation of gingival damage by toothbrushing. An experimental study in dog. Acta Odontol Scand. 1975;33(3):119-27.
5. Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962 Jul;65:26-9.
6. Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970 Jan;41(1):41-3.
7. Lobene RR, Soparkar PM, Newman MB. Use of dental floss. Effect on plaque and gingivitis. Clin Prev Dent. 1982 Feb;4(1):5-8.
8. Paraskevas S, Rosema NA, Versteeg P, et al. The additional effect of a dentifrice on the instant efficacy of toothbrushing: a crossover study. J Periodontol. 2007 Jun;78(6):1011-6.
9. Loe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand. 1963 Dec;21:533-51.
10. Van der Weijden GA, Timmerman MF, Nijboer A, et al. Comparison of different approaches to assess bleeding on probing as indicators of gingivitis. J Clin Periodontol. 1994 Oct;21(9):589-94.
11. Noordzij M, Tripepi G, Dekker FW, et al. Sample size calculations: basic principles and common pitfalls. Nephrol Dial Transplant. 2010 May;25(5):1388-93.
12. Jackson MA, Kellett M, Worthington HV, Clerehugh V. Comparison of interdental cleaning methods: a randomized controlled trial. J Periodontol. 2006 Aug;77(8):1421-9.
13. Bowen DM. Flossing or alternative interdental aids? J Dent Hyg 2012 Spring; 86(2):58-62.
14. Kotsakis GA, Lian Q, Ioannou AL, et al. A network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. J Periodontol 2018 May; 89(5): 558-70
15. Yost KG, Mallatt ME, Liebman J. Interproximal gingivitis and plaque reduction by four interdental products. J Clin Dent. 2006;17(3):79-83.
16. Abouassi T, Woelber JP, Holst K, et al. Clinical efficacy and patients' acceptance of a rubber interdental bristle. A randomized controlled trial. Clin Oral Investig. 2014 Sep;18(7):1873-80.
17. de Souza PH, de Toledo BE, Rapp GE, et al. Reliability of bleeding and non-bleeding on probing to gingival histo-logical features. J Int Acad Periodontol. 2003 Jul;5(3):71-6.