You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Sleep dentistry has primarily focused on the fabrication of a plastic orthotic to protrude the mandible and control obstructive airway events during sleep. Typically, affected patients have a documented history of obstructive sleep apnea (OSA) and concomitant medical complications. Patients routinely present with confounding physical limitations to airway resolution, including but not limited to obesity, nasal incompetence, genioglossal inactivity, and reduced oral volume. In addition, many sleep dentistry patients have failed continuous positive airway pressure (CPAP) titration. The goal of sleep dentistry has been to reduce the number of obstructive events per hour through vertical and protrusive movement of the mandible. If this goal is achieved, the patient is placed on yearly recall to monitor occlusal change, OSA recidivism, and appliance deterioration. If the goal cannot be obtained, the patient is customarily returned to the sleep physician for another attempt at positive airway pressure.
This business-like approach ignores four important issues: (1) Children, whose health is the most damaged by poor breathing, do not fit the sleep dentistry model. Neurocognitive, systemic, and craniofacial alteration during key developmental windows place a premium on diagnosis and resolution of breathing abnormalities as soon as possible. (2) Many individuals with symptoms who fall below the sleep-medicine guidelines for mild OSA receive no treatment. Inspiratory flow limitations (IFLs) and respiratory effort-related arousals (RERAs) are abnormal upper airway functions during sleep that do not score as apnea in a polysomnogram (PSG). Yet they create stress on the neural and neuroendocrine systems and precipitate many of the somatic and systemic issues found in airway patients. (3) A poor airway is a full-time problem. It is accentuated when the person’s wakefulness drive and respiratory neural drive lose responsiveness during sleep. However, patients with OSA require significantly more sympathetic muscular drive of upper respiratory muscles during wakefulness to maintain a patent airway. (4) Pharyngeal collapse is characteristically related to an anatomically small airway. Yet, in the sleep dentistry model, resolution strategies for this deficiency are seldom reviewed with the patient.
This article will discuss the shortsightedness of this conventional strategy for handling a universal health crisis and provide a protocol for improving control with the use of mandibular advancement appliances (MAAs). It will also present an algorithm for interdisciplinary treatment strategies as a possible alternative after or in place of oral appliance therapy.
Recognizing a Sleep Dentistry Patient
In healthcare, a widespread conviction holds that any problem linked to sleep must be explained by apneas/hypopneas causing periodic desaturations and fragmentations. This platform omits most patients who are screened in a dental practice. Usually, an airway patient whom a general dentist sees is not the stereotypical overweight older man who has experienced apnea events. More often, patients will present with malocclusion, narrow dental arches, a posteriorly displaced maxilla, and a retrognathic mandible.1
They may demonstrate wear from bruxism, complain of headaches and/or nasal congestion, and have palpable orofacial muscular pain. The patient’s tongue will routinely appear too large for the arch, sit low in the throat, may look like a scallop around the teeth, and may be restricted in its movement. Many of these patients will be poor sleepers who find it difficult to fall asleep, stay asleep, and/or lie without movement.
Airway patients routinely experience upper airway resistance syndrome (UARS) or ultra-mild sleep apnea. UARS is defined as having an apnea–hypopnea index (AHI) value of less than 5/hr and RERAs greater than 5/hr with associated fatigue.2 These individuals experience IFLs throughout non-REM sleep and possess an upper-airway collapsibility that registers between normal subjects and those with mild-to-moderate OSA.3 In some respects, UARS is an extension of mild OSA with less severe pharyngeal collapse; however, that link does not explain the excessive fatigue, functional somatic disorders, and increasing frequency of sleep-onset/sleep-maintenance insomnia found with UARS.4 Finally, UARS has also shown to be a progressive disease worsening to OSA with advancing age, hormone reduction, and increasing body mass.5
Exploring Chronic Stress
Recent studies explore a new chronic “stress” paradigm rather than the apnea/hypopnea model to explain damage from breathing-disturbed sleep and the progressive nature of the disease.6,7 In this theory, recurrent IFLs are a chronic nidus of stress on the autonomic nervous system (ANS) and hypothalamo-pituitary-adrenocortical (HPA) axis.8 The brainstem receives inputs on real or anticipated disruptions in homeostasis, such as respiratory restriction. The sympathetic nervous system provides the immediate “fight or flight” response to airway limitations. The sympathetic system is centered in the adrenal medulla and uses adrenaline for chemical transmission for escape. The parasympathetic branch is centered in the adrenal cortex and is the “rest and digest” part of the ANS. It uses acetylcholine, nitric oxide, and cyclic guanosine monophosphate as neurotransmitters.9 The parasympathetic and sympathetic divisions incite rapid adjustments in physiologic states through direct neural innervation of end-organ systems. Sympathetic stimulation, with its catecholamine release, is quickly balanced due to a responsive parasympathetic activation. When the balancing activity of the parasympathetic nervous system is lost or reduced due to chronic stressors, the sympathetic reaction to stress cannot be normalized. The result is an uncontrolled increase of adrenaline.
Nightly stress from an inadequate inspiratory flow during sleep can physically alter the brain regions responsible for management of the ANS.9 The habituation of stress responses can lead to long-term changes in autonomic function, including increased heart rate10 and decreased heart rate variability (HRV).11 These surges in sympathetic activity can create a dysautonomia that can be monitored through HRV analysis. Cardiac autonomic modulations can be quantified by evaluating the R-R interval in an electrocardiogram recording. Autonomic dysfunction is seen in increased low-frequency (LF) modulations (sympathetic), decreased high-frequency (HF) modulations (mostly parasympathetic), and increased LF/HF ratio during sleep (ratio of sympathetic to parasympathetic sleep). Quality sleep in adults is comprised of approximately two times more HF sleep than LF sleep, and four times more in children. RERAs and IFLs increase sympathetic tone during sleep even with a low or normal AHI value. The increase in LF power is significantly higher in women than in men, possibly explaining the higher prevalence of chronic stress-related symptoms.12
CPAP therapy has been shown to normalize the LF/HF ratio. The sympathovagal imbalance is reduced13 and the parasympathetic cardiac modulation restored in patients with OSA using CPAP therapy long term.14 The implication is that by reducing the chronic stress of nocturnal inspiratory flow disruption during sleep, the risks from cardiac autonomic nervous system dysfunction may be lowered.12
In addition to the ANS, brainstem signaling of respiratory distress triggers the HPA axis through pathways projecting to the hypothalamus. The HPA axis response is more prolonged and comes from a rise in circulating glucocorticoids.9 The stress response to a physical and psychologic threat can cause a significant rise in adrenal steroid hormone release. The brain levels reach a maximum 50 minutes after the event, with plasma levels rising somewhat quicker. These levels are maintained for a prolonged time. Clearance can take approximately 110 minutes.15 HPA axis steroid hormones regulate the metabolism of glucose and are produced in the adrenal cortex. The composition allows them to reduce inflammation and cause immunosuppression. Cortisol is an important stress-related glucocorticoid. The IFL associated with UARS and mild OSA creates a chronic stress reaction activating the HPA axis. The activation may lead to development of functional somatic syndromes (ie, chronic fatigue, irritable bowl syndrome, fibromyalgia, migraines, tempromandibular disorder) and anxiety disorders. These maladies are significantly more widespread in UARS and mild OSA than in more severe sleep-disordered breathing.16 In addition, these disorders, including insomnia, respond to stabilization of the upper airway with CPAP therapy and rapid palatal expansion.17 The HPA axis recovery involves creating airway patency and reduction of stress during sleep. The recovery may take several nights as sleep consolidates.
Nasal Breathing
It has been more than 40 years since Cottle18 suggested that sleep patterns are in great measure dependent on good nasal function. Smooth laminar flow provided by nasal breathing is more conducive to improved sleep. Airflow receptors in the nose assist in maintaining muscle tone in the oropharynx, creating a patent airway.19 In addition, nitric oxide release is stimulated in the paranasal sinuses by flow of air over the turbinates. Nitric oxide dilates blood vessels, reduces swelling/congestion, and improves lung oxygen transfer. It is also an endogenous antimicrobial agent, killing bacteria and reducing infection. Moreover, nasal disuse and mouth breathing are functionally abnormal and can lead to alterations in craniofacial growth and development. These changes can make people more prone to development of sleep-disordered breathing years later.20
Obstructive apnea and hypopnea occur significantly more frequently when breathing orally (AHI 43 + 6) than nasally (AHI 1.5 + 0.5).21 Promoting nasal breathing through the use of decongestants and external dilator strips can have a dramatic effect on sleep. Subjects with moderate OSA, significant nasal turbinate obstruction, and normal retroglossal airway dimension showed an average AHI reduction of 12 events per hour, improved sleep efficiency, and increased REM and stage-3 non-REM sleep compared to placebo controls.22 In addition, abnormal nasal structures will significantly increase the number of apnea events due to a sizable increase in flow-limited breathing.23 Besides OSA, UARS is also primarily a nasal issue, as demonstrated in a recent report.24 Participants with UARS exhibited an increased frequency of “altered nose,” ie, septal deviation, inferior turbinate hypertrophy, or other airflow obstruction, when compared to controls. Furthermore, patients with UARS had more complaints of oropharyngeal dryness, which is indicative of oral breathing during sleep. The treatment for these patients is not complete until nasal breathing has been fully recovered during both day and night.
Autonomic Nervous System Trial
The autonomic nervous system (ANS) trial was initially designed as an MAA test to reduce the rate of oral appliance failures, both due to anatomic and psychologic causes. In the study, a provisional appliance was utilized for 3 months to determine if the device would be worn, would not cause pain, and would improve the participants’ sleep symptoms. At the end, patients requesting final appliances were fitted for new devices. Many patients who had previously failed CPAP therapy and could not use this device were left without a strategy to control their airways.
The impetus behind the ANS trial began with an understanding that the dysautonomia related to repeated airway stress can eventually damage the responsiveness of the genioglossus. One-third of patients with OSA have minimal or no neural activation during airway challenges.25 These patients will be more difficult to control and will require more aggressive intervention strategies for resolution. This, however, leaves a substantial portion of the OSA population and all patients with UARS still in possession of a large neural drive during sleep. The problem is that the signal fails to resolve the collapse due to an anatomic insufficiency. The ANS trial is an attempt to determine a patient’s neural drive before a final MAA is produced and to use the information obtained to create an interdisciplinary resolution strategy. Failure in the ANS trial does not leave the patient without a strategy but, in fact, highlights possible resolution plans.
Step 1: Evaluate Nasal Airway Patency
Breathing through the mouth causes nasal inflammation, tissue swelling, and mucus secretion. A blocked nose promotes continued mouth breathing and a cycle of dysfunction. Nasal symptoms such as stuffiness, snoring, a poor sense of smell, and difficulty breathing through the nose were reduced 70% with the introduction of nasal breathing exercises.26 Decongesting before sleep with a saline or xylitol rinse and using a nasal unblocking exercise will open the nose, even with the common cold. McKeown27 described a nose-unblocking exercise that entails taking small silent breaths through the nose. On exhalation, individuals pinch their noses to hold their breath. They then walk as many paces as possible while holding their breath, trying to build up a medium-to-strong air shortage without overdoing it. The author has found walking to not be a necessary component of the exercise. It can, therefore, be performed sitting in bed. When resuming breathing, they do so only through the nose and calm the breathing quickly. They then wait 1 to 2 minutes before repeating the breath hold, and repeat for six breath holds, creating a fairly strong need for air. By holding their breath after an exhalation, the unblocking exercise will increase the concentration of nitric oxide in the nasal cavity and will, thus, result in the dilation of the nasal passages.
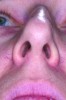
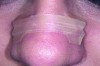
Nasal anatomy may also prevent a patient from breathing efficiently through the nose. A deviated septum may make nasal breathing difficult but rarely prevents completion of the ANS trial. One anatomic factor that could, however, is nasal valve stenosis. The nasal valves must resist collapse to allow normal airflow. The external valves comprise the sidewalls of the nose and nares. The internal nasal valve is a naturally occurring narrow area of the inner nose. The shape is comprised of the upper lateral cartilage, nasal septum, and turbinates. During inspiration, these structures should not collapse or constrict. If they do (Figure 1), dilator strips or internal nasal props can be used to stent the nasal valves (Figure 2). Surgical scaffolding is also a possibility.
Step 2: Buteyko Mouth Taping
One goal of the ANS trial is to extend the advantages of nasal breathing to sleep. The problem is that patients are not aware whether their mouths are open when sleeping. To ensure nasal breathing, the patient can implement Buteyko mouth taping.27 This is done by applying 1-inch paper tape (eg, 3M™ Micropore™, 3M, www.3m.com) across the lips to keep the mouth from falling open during sleep (Figure 3). Folding over the ends of the tape allows it to be removed a bit easier, as the adhesive can make morning removal difficult. Removing the tape can be enhanced further by initially applying lip balm, patting the tape on some cloth before placing it to lessen the strength of the adhesive, or by using a warm, wet cloth in the morning to loosen the tape.
People who have been mouth breathing their entire lives may be apprehensive about mouth taping. To make patients more comfortable with the concept, clinicians should demonstrate the nasal unblocking exercise and proper placement of dilator strips or nasal cones. The tape is applied for the first time to the patient’s mouth in the dental office, and he or she reclines quietly in a dental chair for a minimum of 10 minutes before ending the exercise. It is quite normal for patients to unknowingly remove the tape in their sleep for the first few nights while they reset the breathing center. The brainstem trigger for breathing must be retrained to adjust to a more normal breathing volume. Wearing the tape all night is an opportune way for patients to retrain their respiratory center, but the tape may also be worn during wakefulness, including during light exercise.
It has been proposed27 that taping for 3 to 6 months will be needed to eliminate mouth breathing during sleep once the tape is no longer utilized. The author has not found this to be the case unless the anatomic points of airway resistance are addressed. The ANS trial requires a minimum of 10 days of successful mouth taping before proceeding further. Mouth taping and wakeful breathing exercises, under the guidance of a trained therapist, are appropriate and recommended for children aged 5 years and older.
Step 3: Provisional MAA
If the patient successfully completes the 10 days of mouth taping but is still experiencing symptoms, a provisional MAA will be introduced. In the ANS trial, no patient receives a final MAA until success with a provisional appliance has been achieved. Success is defined as: (1) the patient wears the appliance nightly; (2) use of the appliance does not make muscles and joints uncomfortable; and (3) the patient reports that his or her sleep is improved. The provisional appliance is not titrated with home monitors or a PSG because the design of the final appliance will be different from the provisional and will, thus, alter the results. Titration is reserved for the final appliance.
The provisional oral appliance used in the ANS trial is an irradiated thermoplastic device (Figure 4) with a design that allows it to be modified to almost any arch size and configuration. Vertical adjustment shims are available that enable easy, predictable modification of oral volume. The initial protrusion is set at no more than 2 mm to 3 mm for the first week. Because the appliance breaks the patient’s lip seal, it may introduce unwanted oral breathing (Figure 5). Therefore, mouth tape or an oral shield should be used with the appliance. If the initial protrusion position does not improve sleep after the first week, a routine MAA titration sequence is employed until subjective success is achieved (Figure 6). The ANS trial lasts no longer than 3 months. At each step, both success and failure are valuable outcomes.
Step 4: Control
The chronic stress of upper airway collapses must be controlled to allow healing. If the patient’s airway is stabilized with a provisional appliance, a final MAA is warranted. If the patient will not or cannot wear, or gains no value from wearing, the provisional appliance, a final MAA is not fabricated. Alternative solutions must be pursued to control the nightly stressors.
Step 5: Resolve
In addition to controlling the airway, the ANS trial allows patients to demonstrate their unique level of autonomic dysfunction. This information will aid in planning an interdisciplinary resolution strategy.
The ANS trial differs from sleep dentistry by attempting to resolve the deficit, even if the resolution is successful but not curative. The ear, nose, and throat (ENT)/OSA surgical literature established the foundation for this concept. Historically, attempts to surgically resolve OSA have had limited success. Uvulopalatopharyngoplasty (UPPP) removes excessive palatal tissue and reshapes a collapsible portion of the airway. Its effectiveness in reducing apnea in isolation was limited, reducing the AHI value to <10 in only 33% of the patients.28 Interestingly, Weaver et al29 reported that even though the AHI of patients receiving UPPP did not drop as much as the CPAP therapy subjects, the survival after 12 years was significantly higher in the UPPP group. One obvious explanation for this is the low compliance rate for CPAP therapy, but it also appears that the surgical treatment slowed or halted the progression of the chronic stressor. In addition, the resolution was an improvement of the patients’ full-time airways, not just their sleep airways. The ANS trial conforms to the following concept: Control of the airway is necessary, but resolution is more ideal. The issue with the UPPP surgical approach is that it was being applied haphazardly to patients who had obstructions other than at the soft palate. The ANS trial is a systematic approach to identify areas of obstruction and prospectively simulate a resolution strategy, making surgical, orthodontic, and myofunctional solutions more predictable.
As with any complex dental treatment planning, each airway patient presents a unique set of contributing anatomic, psychologic, and preferential factors that must be addressed. Listing all possible strategies for care here is infeasible; however, the following decision tree will assist in the foundation of a resolution approach.
Patient Unable to Breathe Through Nose All Night—In this case, the referral is to an ENT specialist for nasal evaluation. The ability to breathe comfortably and exclusively through the nose is paramount before any further treatment is attempted. Typically, surgical or allergy treatments are all that is needed to resolve the chronic inspiratory stressor and/or apnea.
Patient Controls and Resolves With Nasal Breathing—In this scenario, the introduction of nasal breathing produces the best night’s sleep the patient has ever experienced. The signs and symptoms of ANS and HPA axis damage resolve or improve significantly. In this case, the patient may simply need assistance with proper nasal breathing in wakefulness and sleep. Breathing instruction and myofunctional therapy can aid in resetting the breathing pattern. An ENT evaluation of the nasal and pharyngeal anatomy would be warranted to determine if minor surgical alteration would reduce restrictions, ie, repair a deviated septum. Finally, orthodontics may be appropriate given that expansion to create improved oral cavity volume has been shown to also improve the nasal and pharyngeal airway.30
Nasal Breathing and Minimal MAA Protrusion—The addition of an oral appliance increases oral volume by opening the vertical dimension of occlusion and assists genioglossal activation by slightly protruding the mandible. If the patient finds symptomatic relief with minimal protrusion, it can be assumed that the patient is among the two-thirds of the population whose ANS is attempting to activate the genioglossus during collapse. Because the patient is actively attempting to clear the airway during sleep, breathing/myofunctional therapy paired with ENT and orthodontic intervention may reduce or halt the chronic pattern of collapse by mimicking the changes introduced by the appliance and tape. Myofunctional therapy has demonstrated an AHI decrease of 50% in adults and 62% in children.31 It is an important adjunct to other apnea treatments when the patient still has a functioning autonomic system.31
Nasal Breathing and Maximum MAA Protrusion—Because the protrusion has pushed the anatomic limits, the patient is probably in the one-third group that lacks the neural drive to actively engage in resolving the airway constriction. Traditional orthodontics cannot mimic the amount of protrusion, and isolated ENT surgery will not ensure success. In this case, large orthodontic movements with surgical assistance may be necessary, and a phased surgical approach may be essential. Surgery may move beyond the anatomy to the neurologic. Hypoglossal nerve stimulators may assist these patients with reactivating the damaged genioglossal response. Breathing therapy should be included due to the oral nature of the obstructions, but neural damage to the genioglossus may prevent myofunctional therapy from being predictable. Obesity is a comorbidity for many of these patients. Therefore, weight-loss counseling and bariatric surgery may be included in the interdisciplinary plans.
Nasal Breathing and MAA With No Success—In an ANS trial, all reactions are results. Before the final MAA is fabricated, the clinician should determine if the patient will not wear the appliance, the patient cannot tolerate the appliance, or the appliance does not improve sleep. When CPAP therapy fails, 65% of the time the patient is not provided another alternative.32 The ANS trial demands that patients find a technique to at least control the airway, if not resolved. Failure in the ANS trial should never leave patients without a strategy for managing their health.
Conclusion
The classic models for sleep medicine and sleep dentistry ignore the vast majority of patients in dental practices with breathing-disturbed sleep. The apnea-only approach misses the chronic stress conditions elicited by ANS and HPA axis activation. While CPAP therapy and MAA are excellent tools, they both have poor long-term compliance, especially for patients with mild OSA and UARS. These traditional concepts do not make any effort to improve the patients’ anatomic deficiency and instead are used to manage around the problem. The ANS trial addresses airway patency through nasal breathing and reduces many of the problems associated with MAA delivery and titration. Finally, patients completing the Trial are always provided an interdisciplinary strategy for attempting to resolve their airway compromise, both day and night.
Disclosure
The author had no disclosures to report.
About the Author
Jeffrey S. Rouse, DDS
Private Practice
San Antonio, Texas
Seattle, Washington
Adjunct Assistant Professor
Department of Prosthodontics
University of Texas Health Science Center at San Antonio
San Antonio, Texas
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Tantrakul V, Park CS, Guilleminault C. Sleep-disordered breathing in premenopausal women: differences between younger (less than 30 years old) and older women. Sleep Med. 2012;13(6):656-662.
2. Guilleminault C, Stoohs R, Clerk A, et al. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781-787.
3. Rees K, Kingshott RN, Wraith PK, Douglas NJ. Frequency and significance of increased upper airway resistance during sleep. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1210-1214.
4. Gold AR, Gold MS, Harris KW, et al. Hypersomnolence, insomnia and the pathophysiology of upper airway resistance syndrome. Sleep Med. 2008;9(6):675-683.
5. Jonczak L, Pływaczewski R, Sliwiński P, et al. Evolution of upper airway resistance syndrome. J Sleep Res. 2009;18(3):337-341.
6. Gold MS, Amdo T, Hasaneen N, Gold AR. Somatic arousal and sleepiness/fatigue among patients with sleep-disordered breathing. Sleep Breath. 2016 Jan 6. [Epub ahead of print]
7. Broderick JE, Gold MS, Amin MM, Gold AR. The association of somatic arousal with the symptoms of upper airway resistance syndrome. Sleep Med. 2014;15(4):436-443.
8. Gold AR. Functional somatic syndromes, anxiety disorders and the upper airway: a matter of paradigms. Sleep Med Rev. 2011;15(6):389-401.
9. Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397-409.
10. Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282(5):R1333-R1341.
11. Farah VM, Joaquim LF, Bernatova I, Morris M. Acute and chronic stress influence blood pressure variability in mice. Physiol Behav. 2004;83(1):135-142.
12. Chandra S, Sica AL, Wang J, et al. Respiratory effort-related arousals contribute to sympathetic modulation of heart rate variability. Sleep Breath. 2013;17(4):1193-1200.
13. Kufoy E, Palma JA, Lopez J, et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One. 2012;7(3):e33769.
14. Palma JA, Iriarte J, Fernandez S, et al. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Auton Res. 2015;25(4):225-232.
15. Droste SK, de Groote L, Atkinson HC, et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149(7):3244-3253.
16. Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123(1):87-95.
17. Miller P, Iyer M, Gold AR. Treatment resistant adolescent depression with upper airway resistance syndrome treated with rapid palatal expansion: a case report. J Med Case Rep. 2012;6:415.
18. Cottle MH. Clinical benefits and disorders following nasal surgery. South Med J. 1968;61(12):1281-1286.
19. White DP, Cadieux RJ, Lombard RM, et al. The effects of nasal anesthesia on breathing during sleep. Am Rev Respir Dis. 1985;132(5):972-975.
20. Guilleminault C, Akhtar F. Pediatric sleep-disordered breathing: new evidence on its development. Sleep Med Rev. 2015;24:46-56.
21. Fitzpatrick MF, McLean H, Urton AM, et al. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur Respir J. 2003;22(5):827-832.
22. McLean HA, Urton AM, Driver HS, et al. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005;25(3):521-527.
23. de Godoy LB, Palombini LO, Martinho Haddad FL, et al. New insights on the pathophysiology of inspiratory flow limitation during sleep. Lung. 2015;193(3):387-392.
24. de Oliveira PW, Gregorio LL, Silva RS, et al. Orofacial-cervical alterations in individuals with upper airway resistance syndrome. Braz J Otorhinolaryngol. 2015 Nov 6. pii: S1808-8694(15)00188-3.
25. Eckert DJ, Gandevia SC. The human upper airway: more than a floppy tube. J Appl Physiol. 2014;116(3):288-290.
26. Adelola OA, Oosthuiven JC, Fenton JE. Role of Buteyko breathing technique in asthmatics with nasal symptoms. Clin Otolaryngol. 2013;38(2):190-191.
27. McKeown P. The Oxygen Advantage. New York, NY: HarperCollins Publishers; 2015:61-66.
28. Khan A, Ramar K, Maddirala S, et al. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the mayo clinic experience. Mayo Clin Proc. 2009;84(9):795-800.
29. Weaver EM, Maynard C, Yueh B. Survival of veterans with sleep apnea: continuous positive airway pressure versus surgery. Otolaryngol Head Neck Surg. 2004;130(6):659-665.
30. Iwasaki T, Yamasaki Y. Relation between maxillofacial form and respiratory disorders in children. Sleep and Biological Rhythms. 2014;12(1):2-11.
31. Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38(5):669-675.
32. Russell JO, Gales J, Bae C, Kominsky A. Referral patterns and positive airway pressure adherence upon diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2015;153(5):881-887.