You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Dental caries is one of the most prevalent diseases worldwide.1,2 Although incipient dental caries can be arrested or even reversed, limited awareness and lack of preventive care often preclude such options. For this reason, dental restorative procedures have become the most prevailing treatment for dental caries.3 The direct Class II composite restoration is one of the most common restorative procedures in dentistry; 45% of direct restorations are Class II.4 Yet Class II direct resin restorations can be one of the most challenging and underpaid procedures.
The strategy of cavity preparation, along with the selection and placement of restorative materials, are vital to short-term and long-term success of the restoration.5 Successful resin composite restorations can be achieved only when the characteristics and limitations of these materials are understood and taken into consideration.
This article will briefly describe the benefits and drawbacks of resin composites. It will then go through the process of a Class II restoration, including diagnosis, caries removal, liner placement, bonding, matrix systems, and polishing.
Resin Composites
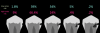
Composite resins were developed in the 1950s as a bisphenol A-glycidyl methacrylate (bis-GMA) resin reinforced with silanated 150-µm ground glass filler particles. Over the course of the following 70 years, the major developments in the formulation of dental composites were reductions in the size of the filler particles, with most composites now containing all submicron-sized filler particles.6 Smaller filler particles allow a higher concentration of fillers to be added to a composite, resulting in better mechanical properties and lower shrinkage stress. Smaller filler particles also improve the esthetic properties of dental materials because the individual fillers are smaller than the wavelength of light and cannot be seen with the eye when the composite is polished.6 The most recent major developments in dental composites have been the introduction of bulk-filled composites, which are formulated as either modifying the composite resin or modifying the translucency of the composite.7 Figure 1 shows the translucency of several bulk-filled composites.
Diagnosis
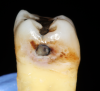
Cavitation of enamel is often used as the threshold for determining whether caries should be treated by a surgical approach; a cavitation may harbor a cariogenic biofilm, protecting it from mechanical cleaning.8 Because interproximal lesions are not typically visible to the clinician, the diagnosis of this cavitation is typically done radiographically, and the clinician must decide which radiographic presentation corresponds to cavitation of enamel (Figure 2). Several clinical studies have attempted to determine a correlation between radiographic depth of a lesion and the presence of enamel cavitation.9 Different methods were used to determine whether interproximal cavitation was present. Some studies assessed cavitation during the preparation by observing the enamel wall through the preparation. In these studies, 52% to 78% of the lesions that had radiographic presentation of caries in the outer third of dentin were determined to be cavitated.9 In other studies, direct observation of cavitation was performed by separating the contact with elastics. In these studies, 28% to 100% of the lesions that displayed radiographic evidence of caries were determined to be cavitated.9
Aside from determining the presence of cavitation, the clinician should consider the activity of the lesion. According to the International Caries Classification and Management System (ICCMS), an active lesion should be treated more aggressively than an inactive lesion. Visual changes seen in enamel can give clues to the activity of a lesion. White demineralization bands, dark shadowing, and frank cavitation are all signs of an active lesion.10
Ultimately there will always be a level of subjectivity and clinician personal bias when planning surgical treatment of a carious lesion. To provide clinicians a reference on the threshold used by other practicing dentists for surgically entering a tooth, a 2009 study from the US Dental Practice-Based Research Network surveyed 500 dentists on their thresholds for treating caries surgically (Figure 3).11 In high caries-risk patients, 66% of dentists chose to surgically treat lesions that were radiographically observed in enamel, whereas 24% chose to wait until the lesions progressed to the inner third of the dentin. In low caries-risk patients, 39% of dentists would treat the enamel-only lesion surgically, and 54% would wait until it progressed to the inner third of dentin. This survey demonstrates that most practicing dentists surgically intervene with operative treatment before likely cavitation of the enamel.
Resin infiltration is a new concept for treating incipient interproximal caries lesions. In this treatment, a hydrochloric acid is applied to enamel to remove the less porous surface layer, and then an unfilled resin is used to infiltrate the internal enamel porosities through capillary movement. Several clinical trials have been performed to assess the clinical success of this treatment. In a study of 50 children, 62% of untreated interproximal lesions progressed within a year, whereas only 23% of those infiltrated showed radiographic progression.12 In another study with 39 adult patients, 32% of infiltrated, 41% of sealed, and 70% of untreated interproximal lesions showed progression after 3 years.13 In a third study of 29 adult patients, 42% of untreated interproximal lesions progressed within 3 years, whereas only 4% of those infiltrated progressed.14 A recent Cochrane review concluded that resin infiltration significantly reduces the likelihood of caries progression more than noninvasive treatments.14
Caries Removal
The next step is deciding how much caries to remove and what to leave behind. The more tooth the clinician removes during preparation, the less tooth structure will be available in the future should the restoration fail. Replacement of failed restorations accounts for nearly 70% of all restorative dentistry.15 Furthermore, restoration techniques that require a greater amount of healthy tooth to be removed may negatively impact tooth pulp.16,17
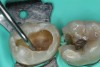
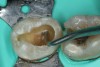
To determine how much to remove, it is important to understand the difference between infected dentin and affected dentin. The infected layer of dentin is highly demineralized, is physiologically unable to remineralize, and contains irreversibly denatured collagen fibrils with a virtual disappearance of cross-linkages.18 It contains bacteria and degraded collagen that cannot be remineralized. On the other hand, the affected dentin has a minimal concentration of bacteria, and the collagen network is still viable.19 Clinically, affected and infected dentin are differentiated by their hardness. Infected dentin is soft and can be easily removed with a spoon excavator (Figure 4). Affected dentin is leathery and requires firm pressure to be removed with a spoon excavator (Figure 5).20
The impetus for leaving affected dentin in the preparation is to prevent direct pulp exposure. Incomplete caries removal leads to 77% less pulpal exposures than complete caries removal.21But bonding to affected dentin provides a lower bond strength than bonding to healthy enamel.22 Therefore, affected dentin should only be left in the preparation in order to prevent pulpal exposure. Affected dentin is left only on the pulpal floor or axial wall when removal of this caries is expected to expose or nearly expose the pulp (Figure 6). A periphery of sound enamel and dentin should be prepared to create a seal of strongly bonded tooth structure. Affected dentin may be left over at the pulpal floor because a permanent restoration will seal remnant bacteria from their source of nutrition, causing bacterial death or dormancy.23
One important precondition to selective caries removal for deep caries is to assess the preoperative pulpal health of the tooth. Only teeth with a normal pulpal response or reversible pulpitis should be attempted for direct restorative treatment after selective caries removal. Teeth with necrotic pulp or irreversible pulpitis should directly receive root canal treatment.
Liner Placement
The philosophy of selective caries removal is to leave affected dentin in areas of deep caries to prevent pulpal exposure. This avoids the need to perform a direct pulp capping procedure in which a liner is placed directly on the pulpal exposure. Despite an intention to avoid direct exposures, these exposures may still occur unintentionally due to an unexpected location of a pulp horn or iatrogenically due to a desire to remove affected dentin. In the case of a direct pulp exposure, the placement of a liner is necessary because reparative dentin cannot be formed when bonding agent is placed directly on the pulp (Figure 7).24 Two recent clinical trials have compared the survival rates of teeth treated with direct pulp caps with calcium hydroxide or calcium silicate (mineral trioxide aggregate [MTA]). Both trials reported an approximately 80% 3-year survival rate with the calcium silicate material and 50% 3-year survival rate with the calcium hydroxide material.25,26
When performing selective caries removal, the remaining affected dentin (and any healthy dentin potentially left underneath) will act as the protective barrier between the pulp and the restoration. Therefore, it is questionable whether it is necessary to place a liner when using selective caries removal and a direct pulp exposure does not occur. Many clinicians report using liners. About 56% of dentists use liners daily or weekly, whereas only 13% report not using them at all.27 A recent review has suggested that there is no improvement in clinical outcome with the use of calcium hydroxide liners over deep caries. Clinicians still struggle with the concept of leaving potentially living bacteria in affected dentin without a treatment to kill these organisms. Some clinicians choose to use a 2% chlorhexidine rinse on the affected dentin because this solution has proven to be bactericidal.28 A recent trend has been the application of silver diamine fluoride on remaining affected dentin. This author is not aware of any published evaluation of this application of silver diamine fluoride.
Types of Bonding
Adhesive dentistry has undergone great progress in the last decades. Categories of adhesive techniques include etch-and-rinse (total etch) and self-etch.29 Each mode has advantages and disadvantages. The benefit of total etch is that it applies phosphoric acid, with a pH well below 1, to the surface of enamel. This ensures a thorough etch pattern on the surface of enamel. The disadvantage of a total-etch approach is that it has more technique variability because the amount of time for which dentin is etched and the residual wetness of the dentin after rinsing can have a large effect on the bond to dentin. The benefit of self-etch is that it takes away some of the technique sensitivity of etching dentin with phosphoric acid. However, the disadvantage is that some self-etch adhesives are not acidic enough to create surface texture on enamel (Figure 8 and Figure 9).30 In order to overcome this disadvantage, self-etch adhesive can be used in a selective-etch mode, which is application of phosphoric acid on the enamel and self-etch application on the dentin (Figure 10). Another perceived benefit of self-etch bonding systems over total-etch bonding systems is the incidence of postoperative sensitivity. There have been several recent reviews of clinical trials that showed no difference in postoperative sensitivity between self-etch and total-etch adhesives; however, clinical trials often compare ideal clinical situations with shallow to medium-sized restorations.31 In a practice-based research network study, there was a slightly lower incidence of postoperative sensitivity with self-etch adhesives.31
Reviewing the clinical trials that have compared these bonding modes, it is apparent that both modes can have excellent clinical performance when two-bottle systems are used. Two systematic reviews performed by Peumans et al confirmed that two-bottle adhesives outperform single-bottle systems.32,33 A recent exception to this rule may be the performance of universal adhesives, which are mildly acidic single-bottle systems. When these adhesives are used in a total-etch or selective-etch mode (etching enamel only), they appear to perform similarly to some two-bottle systems.30
Despite the clinical success of two-bottle systems, a 2019 survey of US dentists reported that 72% use single-bottle adhesives systems.34 There are some potential benefits of using single-bottle systems. Because there is only one step, there is less time for which the clinician must keep the area isolated. The use of a rubber dam would eliminate this issue; however, the reality is that only 37% of clinicians ever use rubber dams for operative procedures, and rubber dams are only used for about 12% of restorations overall.35 Another potential benefit is that fewer steps lead to less confusion in following the bonding protocol.
Matrix
Isolation with a matrix system can be a challenge with Class II composite restorations. Not only must the selected matrix system seal the preparation to help achieve marginal integrity, it must mimic natural tooth contour and facilitate interproximal contact.36 To overcome the challenges when placing a Class II composite resin to achieve an anatomically formed proximal contact, specialized matrix systems have been introduced (Figure 11). Composite resin, unlike dental silver amalgam, is not packable and cannot move a matrix band to achieve an anatomic proximal contact. Composite resin by its chemistry is a viscous liquid that may be moved and displaced but not made more dense during placement.37,38
To address this problem, dentists and manufacturers have designed specialized matrix systems that allow the clinician to achieve an anatomic proximal contact. Thin, deadsoft, stainless steel matrices (0.001-inch thickness) for use with a Tofflemire retainer and sectional matrices (0.001-inch) to be used with metal, spring-like rings provide advantages over thicker, more rigid stainless steel matrices (0.002-inch and 0.0015-inch) used for dental silver amalgam placement. Placing a deadsoft matrix can be a challenge if the proximal contact is not completely separated with the preparation. In this case, it may be advantageous to use a thicker matrix band. These ring systems, some with enhanced silicone or composite wings, have the advantage of providing additional wedging of teeth, creating separation to compensate for thickness of the matrix band to ensure good proximal contact. They allow better contouring on the facial and lingual surfaces-especially when the preparation extends beyond the tooth-line angles-providing a more anatomic contour, with less excess that would need finishing. They are especially useful for single proximal surface placement when compared with the use of a circumferential band.37,38
Composite Resin Placement
The placement of posterior composite resins has been recommended to be performed using an incremental layering placement technique. In this technique, each layer was recommended to be 2 mm to achieve sufficient depth of cure. The theory behind this technique was that by placing 2-mm increments, it would reduce the volumetric polymerization shrinkage stresses of the composite by providing more unbonded surface area per increment. However, this theory has come into question.39 There has been concern that incremental placement of posterior composite resin, especially Class IIs, can lead to voids within the composite. In recent years, a new class of posterior composite resins, bulk fill, has been introduced that provides low polymerization shrinkage and 4-mm depth of cure.40 The depth of cure of these bulk-fill composites has been verified; however, there is less evidence to prove that shrinkage stress has been improved with new formulations of bulk-fill composites.7 One strategy to take advantage of the depth of cure of bulk-fill composites is to continue to place them in increments, realizing that it is difficult to estimate 2 mm clinically, so some of the increments may be larger than 2 mm.
Final Steps
Polishing a composite is performed more than just for its appearance. Some studies have shown that a patient's tongue can detect roughness on the scale of 0.5 µm.41 If the composite has a roughness value above 0.2 µm, it will be susceptible to plaque accumulation.42 Additionally, a polished composite is more stain resistant.43
Before polishing, the restoration is typically finished with either finishing carbides or diamonds. A recent study found a 10-fold increase in roughness using a 20-grit diamond finishing bur (Ra = 2.5 µm) compared with a 30-blade tungsten carbide finishing bur (Ra = 0.25 µm).44 After the shaping and smoothing of restorations that occurs with a bur, they are then polished. There are various shapes and formulations of composite finishing and polishing tips that typically contain either aluminum oxide or diamond abrasives (Figure 12).
One of the more well-known systems is composed of the Enhance® finishing (first step) and Enhance PoGo® polishing (second step) tips (Dentsply Sirona, dentsplysirona.com). In this system, the abrasive particles are embedded in a urethane dimethacrylate binder, which allows these tips to finish aggressively with high pressure and then smoothly with lower pressure; however, the high pressure can cause wear on the binder, limiting the lifetime of these tips. Other points use rubber-based binders (OptraPol®, Ivoclar Vivadent, ivoclarvivadent.com; Jiffy™, Ultradent, ultradent.com); in this author's experience, these polishers maintain their shape for a longer period of time than the former product, although they do not allow for aggressive finishing with increased pressure.
Flexible polishing discs are commonly used for anterior composites (eg, Sof-Lex™ contouring and polishing discs, 3M ESPE, 3m.com; FlexiDisc®, Cosmedent, cosmedent.com). They contain aluminum oxide particles of different sizes, ranging from 5 µm to 100 µm. Several studies have compared the polish achieved with flexible polishing discs to that of polishing points, with some studies showing an advantage with the points44 and others with the discs.45 The advantage of polishing discs in anterior restorations is that they are flexible and can adapt to embrasure spaces.
Another type of polisher is those that are spiral shaped (eg, Sof-Lex Diamond, 3M ESPE; DiaComp Feather Lite™, Brasseler USA, brasselerusadental.com; Footsie™ composite polisher, Komet USA, kometusa.com). A recent study determined that spiral-shaped polishers were able to achieve a smoother surface than several polishing points.46 In this author's experience, the spiral-shaped polishers achieve a very high gloss; however, the surface must be prepolished because these types of polishers do not allow for gross adjustment of the composite surface.
Conclusion
Dental caries, or tooth decay, is one of the most prevalent diseases, affecting about 97% of the population worldwide during their lifetimes.47 When addressing caries with a Class II composite restoration, the goal is to provide tight contacts, good contours, and proper anatomy. Each phase in the restorative procedure should be meticulously implemented to ensure the long-term success of the restorations.
About the Author
Nathaniel Lawson, DMD, PhD
Assistant Professor, Department of Clinical and Community Sciences, Division of Biomaterials, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
References
1. Bernabé E, Sheiham A, Sabbah W. Income, income inequality, dental caries and dental care levels: an ecological study in rich countries. Caries Res. 2009;43(4):294-301.
2. Joves GJ, Inoue G, Sadr A, et al. Nanoindentation hardness of intertubular dentin in sound, demineralized and natural caries-affected dentin. J Mech Behav Biomed Mater. 2014;32:39-45.
3. Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65(10):1028-1037.
4. American Dental Association. Procedure Recap Report. Chicago, IL: American Dental Association; 2006.
5. Chuang SF, Chang CH, Chen TY. Contraction behaviors of dental composite restorations-finite element investigation with DIC validation. J Mech Behav Biomed Mater. 2011;4(8):2138-2149.
6. Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27(1):29-38.
7.Van Ende A, De Munck J, Lise DP, Van Meerbeek B. Bulk-fill composites: a review of the current literature. J Adhes Dent. 2017;19(2):95-109.
8. Wenzel A. Radiographic display of carious lesions and cavitation in approximal surfaces: advantages and drawbacks of conventional and advanced modalities. Acta Odontol Scand. 2014;72(4):251-264.
9. Kidd EA, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. 2004;83(spec no C):C35-38.
10. Ismail AI, Pitts NB, Tellez M; authors of International Caries Classification and Management System (ICCMS). The International Caries Classification and Management System (ICCMS™): an example of a caries management pathway. BMC Oral Health. 2015;15(suppl 1):S9.
11. Gordan VV, Garvan CW, Heft MW, et al; DPBRN Collaborative Group. Restorative treatment thresholds for interproximal primary caries based on radiographic images: findings from the Dental Practice-Based Research Network. Gen Dent. 2009;57(6):654-680.
12. Ekstrand KR, Bakhshandeh A, Martignon S. Treatment of proximal superficial caries lesions on primary molar teeth with resin infiltration and fluoride varnish versus fluoride varnish only: efficacy after 1 year. Caries Res. 2010;44(1):41-46.
13. Martignon S, Ekstrand KR, Gomez J, et al. Infiltrating/sealing proximal caries lesions: a 3-year randomized clinical trial. J Dent Res. 2012;91(3):288-292.
14. Meyer-Lueckel H, Bitter K, Paris S. Randomized controlled clinical trial on proximal caries infiltration: three-year follow-up. Caries Res. 2012;46(6):544-548.
15. Murray PE, Windsor LJ, Smyth TW, et al. Analysis of pulpal reactions to restorative procedures, materials, pulp capping, and future therapies. Crit Rev Oral Biol Med. 2002;13(6):509-520.
16. Thomas MS, Kundabala M. Pulp hyperthermia during tooth preparation: the effect of rotary instruments, lasers, ultrasonic devices, and airborne particle abrasion. J Calif Dent Assoc. 2012;40(9):721-731.
17. Waerhaug J. Presence or absence of plaque on subgingival restorations. Scand J Dent Res. 1975;83(1):193-201.
18. Kuboki Y, Ohgushi K, Fusayama T. Collagen biochemistry of the two layers of carious dentin. J Dent Res. 1977;56(10):1233-1237.
19. Nakornchai S, Atsawasuwan P, Kitamura E, et al. Partial biochemical characterisation of collagen in carious dentin of human primary teeth. Arch Oral Biol. 2004;49(4):267-273.
20. Innes NP, Frencken JE, Bjørndal L, et al. Managing carious lesions: consensus recommendations on terminology. Adv Dent Res. 2016;28(2):49-57.
21. Ricketts D, Lamont T, Innes NP, et al. Operative caries management in adults and children. Cochrane Database Syst Rev. 2013;(3):CD003808.
22. Yoshiyama M, Tay FR, Torii Y, et al. Resin adhesion to carious dentin. Am J Dent. 2003;16(1):47-52.
23. Thompson V, Craig RG, Curro FA, et al. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139(6):705-712.
24. Nowicka A, Wilk G, Lipski M, et al. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, biodentine, and dentin bonding system in human teeth. J Endod. 2015;41(8):1234-1240.
25. Hilton TJ, Ferracane JL, Mancl L; Northwest Prac-
tice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J Dent Res.
2013;92(7 suppl):16S-22S.
26. Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. 2017;50(10):924-932.
27. ACE Panel Report. Bioactive materials. American Dental Association. https://www.ada.org/~/media/ADA/Science%20and%20Research/Files/ACE_Panel_Report_Bioactive_Materials_Q2_2018.pdf?la=en. Published 2018. Accessed May 14, 2019.
28. Borges FM, de Melo MA, Lima JP, et al. Antimicrobial effect of chlorhexidine digluconate in dentin: in vitro and in situ study. J Conserv Dent. 2012;15(1):22-26.
29. Sofan E, Sofan A, Palaia G, et al. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma). 2017;8(1):1-17.
30. Lawson NC, Robles A, Fu CC, et al. Two-year clinical trial of a universal adhesive in total-etch and self-etch mode in non-carious cervical lesions. J Dent. 2015;43(10):1229-1234.
31. Reis A, Dourado Loguercio A, Schroeder M, et al. Does the adhesive strategy influence the post-operative sensitivity in adult patients with posterior resin composite restorations? A systematic review and meta-analysis. Dent Mater. 2015;31(9):1052-1067.
32. Peumans M, De Munck J, Mine A, Van Meerbeek B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions: a systematic review. Dent Mater. 2014;30(10):1089-1103.
33. Peumans M, Kanumilli P, De Munck J, et al. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21(9):864-881.
34. ACE Panel Report. Bonding agents. American Dental Association. https://www.ada.org/~/media/ADA/Science%20and%20Research/Files/ACE-Panel-Report_Bonding-Agents_Q4-2018.pdf?la=en. Published 2018. Accessed May 14, 2019.
35. Gilbert GH, Litaker MS, Pihlstrom DJ, et al; DPBRN Collaborative Group. Rubber dam use during routine operative dentistry procedures: findings from the Dental PBRN. Oper Dent. 2010;35(5):491-499.
36. Shuman I. Excellence in class II direct composite restorations. Dent Today. 2007;26(4):102-105.
37. Loomans BA, Opdam NJ, Roeters JF, et al. Influence of composite resin consistency and placement technique on proximal contact tightness of Class II restorations. J Adhes Dent. 2006;8(5):305-310.
38. Hilton TJ, Broome JC. Direct posterior esthetic restorations. In: Summitt JB, Robbins JW, Hilton TJ, Schwartz RS, eds. Fundamentals of Operative Dentistry: A Contemporary Approach. 3rd ed. Chicago, IL: Quintessence Publishing; 2006:289-339.
39. Versluis A, Douglas WH, Cross M, Sakaguchi RL. Does an incremental filling technique reduce polymerization shrinkage stresses? J Dent Res. 1996;75(3):871-878.
40. Jang JH, Park SH, Hwang IN. Polymerization shrinkage and depth of cure of bulk-fill resin composites and highly filled flowable resin. Oper Dent. 2015;40(2):172-180.
41. Jones CS, Billington RW, Pearson GJ. The in vivo perception of roughness of restorations. Br Dent J. 2004;196(1):42-31.
42. Bollen C, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13(4):258-269.
43. Barakah HM, Taher NM. Effect of polishing systems on stain susceptibility and surface roughness of nanocomposite resin material. J Prosthet Dent. 2014;112(3):625-631.
44. Daud A, Gray G, Lynch CD, et al. A randomised controlled study on the use of finishing and polishing systems on different resin composites using 3D contact optical profilometry and scanning electron microscopy. J Dent. 2018;71:25-30.
45. Gönülol N, Yilmaz F. The effects of finishing and polishing techniques on surface roughness and color stability of nanocomposites. J Dent. 2012;40(suppl 2):e64-e70.
46. Pala K, Tekçe N, Tuncer S, et al. Evaluation of the surface hardness, roughness, gloss and color of composites after different finishing/polishing treatments and thermocycling using a multitechnique approach. Dent Mater J. 2016;35(2):278-289.
47. Berg JH. The marketplace for new caries management products: dental caries detection and caries management by risk assessment. BMC Oral Health. 2006;6(suppl 1):S6.