You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Unhealthy lifestyle choices contribute to such commonly known global epidemic health problems as obesity and diabetes. However, another health issue that is subtly on the rise is dental erosion. Epidemiologic studies show that 30% of young adults (18-35 years) have at least one tooth with erosive tooth loss.1 Lifestyle choices and eating habits may predispose a person toward the loss of dental structure by corrosive processes.2 Dental erosion, also known as "biocorrosion," is a multifactorial condition associated with an irreversible loss of dental structure brought on by a chemical process involving non-bacterial acid sources. Acidic foods and drinks such as citrus fruits and soda, medications, gastroesophageal reflux disease (GERD), and eating disorders cause demineralizing factors that contribute to a higher incidence of non-carious lesions and dental erosion.3,4
This destructive process is cumulative and weakens the enamel of the tooth, which may lead to considerable surface wear. Various intrinsic and extrinsic factors (eg, acid sources) contribute to dental erosion where high acidic concentrations of hydrogen ions (H+) surrounding dental enamel disrupt the pH balance. This causes a shift in the demineralization-remineralization equilibrium where basic calcium and phosphate concentrations are lowered and, ultimately, demineralized. Furthermore, this erosive process often is combined with mechanical processes such as abrasion caused by oral habits or abrasive substances, including routine use of dentifrices, or attrition caused by tooth-to-tooth contact.5
The combined effects of demineralization, erosion, abrasion, and attrition can contribute to excessive tooth wear. In 2009, the World Health Organization 7th global conference advocated the integration of dental care into primary healthcare services and reliance on the collaborative work of a diverse array of healthcare providers.6 This integrative strategy has the premise that a cluster of modifiable risk factors, whether of intrinsic or extrinsic origin, such as diet and other habits, contributes to oral and non-communicable diseases together. Accordingly, the present review was conducted to discuss existing evidence on preventive strategies for controlling or limiting the erosive process progression. It also provides a summary of current knowledge and ongoing research and the implications of said knowledge and research for preventing dental erosion in its early stages. (Author's note: While this article discusses strategies for the prevention of dental erosion, it is not intended to provide restorative options.)
Diagnosis
Erosion is characterized by chemical dissolution and loss of a micrometric layer of enamel or dentin each time it is contacted by an acidic challenge not caused by bacteria. Its etiology is multifactorial and not fully understood. Erosive wear is caused by the surface being softened by acidic substances of intrinsic and/or extrinsic origins. Therefore, a differential diagnosis, including the patient's account of his or her past medical and dental history, an intraoral physical examination, and complementary tests, is essential to identify causative factors.7,8
Ganss and Lussi have listed indices for diagnosis to differentiate dental erosion from other types of tooth wear.9 Cases of dental erosion may resemble amelogenesis imperfecta (AI); however, AI is characterized by an alteration of enamel in both dentitions, increased sensibility to temperature change, reduced esthetic appearance and discoloration of anterior teeth, and masticatory problems.10
According to the European Federation of Conservative Dentistry, there are patient-related nutritional and occupational factors that must be frequent, constant, and sustained over a long period to cause clinical evidence of dental erosion.7 The main patient-related factors are a possible predisposition of the teeth to erosion, behavioral habits, stomach acid, medications, dietary supplements, and oral hygiene.7
Risk Factors
Intrinsic Factors
Saliva is a protective substance that is naturally available in the oral cavity. It forms an acquired pellicle that acts as a barrier that protects the surface of teeth. In addition, saliva flow and buffering capacity clear and remove acid introduced to the oral environment. Furthermore, saliva contains fluoride, calcium, and phosphate, which can remineralize the tooth surface.11 Patients with reduced salivary flow present a greater risk factor for erosive wear. Low salivary flow decreases the pH environment, making it more acidic and prone to erosion. The pH of resting saliva is approximately 6.7, while the pH of stimulated saliva (7.35) is significantly greater.12 Health professionals should evaluate the salivary flow to identify the causes of why it is reduced and intercede to address the condition.
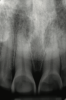
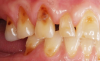
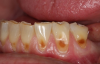
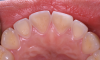
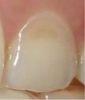
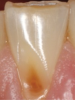
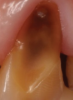
Another intrinsic factor is the patient's gastric acid entering the oral cavity. The source of intrinsic stomach acid may range from rumination or gastroesophageal reflux to chronic alcoholism, pregnancy, or vomiting caused by anorexia nervosa or bulimia nervosa.13 Figure 1 through Figure 3 show the radiographic presentation and clinical examples of dental erosion in advanced stages in the same patient caused by the combination of bulimia nervosa and chronic alcoholism.
Gastric acid significantly demineralizes enamel and dentin, making these structures more susceptible to wear if these medical conditions become chronic. Physicians and dentists should be careful in prescribing medications or agents that have the potential to cause erosion. These include vitamin C,14 iron-tonic and amino-acid supplements,4 aspirin,4 preparations containing hydrogen chloride,4 asthma medications,15 low-pH mouthrinses,4 liquid medications/pediatric syrups,16 acidic salivary substitutes,4 and bleaching agents.17 Some medications may cause erosion by secondary effects such as drug-induced hyposalivation, drug-induced gastroesophageal reflux, and drug-induced vomiting.4
Extrinsic Factors
The consumption of acidic foods and beverages is an extrinsic risk factor. Increased contact with the mouth (ie, through sucking or sipping) may heighten the duration and localization of the clinical erosive effects.18-20 Moreover, the consumption frequency also may elicit erosion.21 Some commercially available beverages have been implicated in chemical erosion.22 Observations showed 93% of tested drinks (including water and sports drinks, fruit juices and drinks, soda, energy drinks, teas, and coffees) had a pH lower than 4, and 39% were considered extremely erosive. The most acidic beverages tested, with a pH lower than 2.4, were lemon juice and various well-known, name-brand colas.22 Water is not categorized as acidic; however, some botted water is, in fact, acidic (pH <7), and some flavored waters have a pH lower than 4.22 Low pH is a critical factor in tooth erosion and is responsible for the immediate demineralization and softening of enamel and dentin structures.
Besides chemical factors, mechanical factors from attrition and abrasion may also have an adverse effect. The use of high-abrasion toothpaste,23 improper brushing technique, and use of whitening toothpaste24 after eating acidic products may be harmful to the dental structure. Patients should be made aware of the abrasion potential of dentifrices they are using. Occlusal wear due to habitual oral actions such as bruxism cause mechanical tooth wear that increases the risk of restorations fracturing.25 This continual tooth wear in combination with erosion can expose the underlying dentin and cause sensitivity and potential pulp exposure.25 Certain somewhat obscure occupations, such as swimming26 and wine tasting,27 also have been identified as etiologic agents in the erosive process. Patients should be questioned on other potential sources of tooth erosion during the review of their past personal, medical, and dental histories.
Clinical Signs
In the early stages of dental erosion, obtaining a diagnosis can be difficult, because few or no signs may be detected. Initial indications of enamel erosive lesions are a smooth, shiny surface with the absence of perikymata and lobi, but the color remains unaltered.8,21,28 Once the condition advances, the morphology changes with the loss of enamel, and color changes may manifest with the tooth appearing increasingly yellow due to the thinning of enamel and shine-through of dentin.
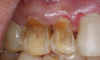
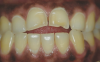
Translucency on the incisal edge of a tooth and intact gingival margin may indicate moderate erosion. In severe cases, the morphology of the tooth becomes significantly altered, which may lead to dentin and/or pulp exposure (Figure 4). The white translucent enamel becomes worn from the surface, leaving yellow dentin and reddish pulp. Worn incisal edges of teeth can be prone to further weakening and fractures. Exposed dentin can cause hypersensitivity to cold, hot, and tactile stimuli.21 If untreated, an exposed pulp may be susceptible to infection and necrosis.
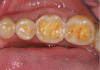
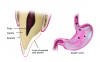
Concavities on the lingual surfaces of maxillary incisors (Figure 5) or occlusal surfaces of premolars and molars (Figure 6) with hollowed areas are signs of dental erosion that may be combined with attrition. Gastric acid is the most common cause of erosion on the lingual aspects of teeth. The most frequent etiology of gastric acid attack is the clinical diagnosis of GERD.13 Patients with anorexia nervosa and bulimia nervosa exhibit higher incidences of dental erosion.29 In Figure 7, a schematic drawing of an upper incisor demonstrates the sequence of loss of dentin and enamel on the lingual/palatal aspect. The direct contact of acid on the tooth structure is the main mechanism as the inorganic material dissolves, leading to a loss of surface hardness.13
The clinician must recognize these clinical signs and characteristics in order to identify erosive lesions in their initial stages so preventive strategies may be implemented as early as possible and enamel and dentin structure preserved.
Methods to Assess Dental Erosion
The diagnosis of erosive wear involves the assessment of lesion characteristics and evaluation of the medical, nutritional, and occupational history of the patient. The Basic Erosive Wear Examination (BEWE) was created to provide a method for quantifying the severity of erosion through a simple scoring system.30 This tooth wear index is reproducible for identifying clinical signs and offers guidance in decision-making for the management of erosive tooth wear. This index recently was validated, supporting its use in clinical practice.31
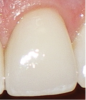
The BEWE is a scoring system that evaluates the most severely affected surface in each sextant, recording one of the four-level scores as follows30: 0 = no erosive tooth wear (Figure 8); 1 = initial loss of surface texture (Figure 9); 2 = distinct defect, hard-tissue loss <50% of surface area (Figure 10); 3 = hard-tissue loss ≥50% of surface area (Figure 11). (Scores 2 and 3 often involve dentin.) After all sextants are assessed, the sum of scores provides the risk level of each patient and may help guide the design of a professional management program for the patient.
From this assessment, appropriate preventive or other intervening treatments can be determined. Furthermore, through the utilization of patient study models and photographs this tooth wear index can be used to help the clinician design a monitoring program in situations involving erosive destruction.
Clinical Prevention Strategies
Erosive tooth wear is an irreversible process. Once this condition has been identified, the health practitioner must determine the main etiological factor(s), strive to eliminate them, monitor the patient, and, if necessary, refer him or her to a dentist for preventive interventions and restorative treatment as needed. As part of the prevention plan, the dentist should educate the patient about the condition and consult with the referring health practitioner. Health professionals should have a broad knowledge of the causative intrinsic and extrinsic factors involving dental erosion, and sufficient information should be gathered from the patient's medical and dental histories so that a personalized preventive strategy may be formulated.
In-office intervention of dental erosion can be regarded in different phases: immediate, interim, and long-term. The immediate phase comprises early diagnosis, monitoring the status of wear, applying preventive strategies of reduced contact with acids, and increasing the resistance of dental structures. Interim and long-term management include provisory and definitive restorations when necessary, recording of wear progression, and modification and reinforcement of preventive action.32
Lifestyle Modifications
In offering preventive instructions to the patient, the health practitioner, be it a physician, dentist, or even dental hygienist, must identify the causal factors of erosive tooth wear, which should be followed by instruction in modifying or avoiding these factors. To address dietary causes, ideally abstinence from acidic foods and drinks would be recommended for prevention of dental erosion. However, if the patient cannot comply, modifying such eating and drinking habits might also suffice. Modifications to reduce the frequency of intake and contact time of acids may slow the progression of dental erosion considerably. The duration of acid contact to the dentition can be decreased through controlled intake whereby patients do not hold or swish acidic beverages in the mouth and avoid slow ingestion of such drinks. Figure 12 is an example of the clinical erosion of enamel of a patient who often sipped and held soda drinks in his mouth in high frequencies throughout the day. This habit promoted loss of enamel and dentin in the facial and incisal surfaces of the patient's upper incisors. He was made aware of how this habit contributed to the tooth pathology and was educated on dietary and habit changes in an effort to reduce tooth destruction.
Another preventive strategy is the consumption of calcium and phosphate mineral-rich products, such as milk, cheese, liver, broccoli,33 and yogurt,20 to promote remineralization. In contrast to low-pH products, higher concentrations of calcium, phosphate, and fluoride ions have a protective influence on tooth wear. By reducing the titratable acidity, products with added calcium, phosphate, and/or fluoride will not demineralize enamel or dentin.21 Despite having a low pH, milk and yogurt have shown to reduce erosive capacity due to high contents of calcium and phosphate.20
Saliva flow is also an important factor that may be modifiable. Patients with xerostomia must be assessed to determine the cause of the condition, such as medication use, lifestyle, or genetics. If the patient's salivary flow is mild, chewing gum may improve flow and encourage mineral precipitation in erosion lesions. Chewing gum containing casein phosphopeptide-amorphous calcium phosphate (CPP-ACP),34 a milk protein derivative, or using CPP-ACP-based toothpaste35 has been reported to have a preventive effect on enamel. CPP-ACP has been shown to reduce demineralization and induce remineralization through the release of free calcium and phosphate ions, maintaining the supersaturated environment.36 In addition, chewing gum in general may help to decrease postprandial esophageal acid contact, reducing erosion caused by intrinsic factors.21 If medications are the cause of xerostomia leading to dental erosion, then the patient must be referred to his or her physician to possibly modify the medications. Additionally, patients with xerostomia may benefit from recommendations for moisturizing the mouth throughout the day.
Medication alterations can greatly affect the progression of dental erosion either directly or indirectly. For example, chewable tablets for the treatment of stomach disorders may contain hydrochloric acid or other forms of acid that bear the risk of directly causing erosion.37 Other forms of medication such as tranquilizers, antihistamines, and antiemetic and antiparkinsonian medicaments can cause salivary gland dysfunction that may further lead to dental erosion if the usage is long term.36 Health professionals should evaluate patients' medications and duration of use to help identify the cause(s) of medication-related dental erosion.
Over-the-Counter Topical Applications
Strategies to prevent erosion also include the application of topical fluorides. Much in vitro and in situ evidence confirms that fluoride-containing dentifrices, varnishes, gels, and solutions may help reduce the progression of dental erosion.38-42 This beneficial effect may be associated with the formation of primarily calcium fluoride-like byproducts or be due to the formation of a physical barrier, which can protect hard dental tissues against acid attack.43
Efforts to develop products that hamper erosion have led to testing with bioactive materials. These materials may possibly stimulate the formation of a calcium-phosphate layer in the dental structure.35 Bakry et al evaluated a 45S5 bioglass paste and showed that it improved the mechanical properties of subsurface-eroded structure in a short treatment through deposition of high quantities of calcium and phosphate on tooth structures.44 Other bioactive products include calcium-silicate materials. Some authors have investigated a novel toothpaste containing calcium silicate, sodium-phosphate salts, and fluoride. These materials have been shown to form hydroxyapatite on the enamel surface, repairing demineralized enamel after exposure to extrinsic acids.45-47
Recent research has focused on other protective agents such as polyvalent metal ions (ie, tin and titanium ions). Stannous fluoride and hydroxyapatite form four main products: stannous hydroxyl phosphate (Sn2OHPO4), stannous fluoride phosphate (Sn3F3PO4), calcium fluoride (Ca(SnF3)2) (usually obtained from tin fluoride with hydroxyapatite for commercially available dentifrices), and calcium fluoride (CaF2) salts. These products produce a stable layer resistant to acid dissolution. Use of tin-containing dentifrices led to 25% to 85% less surface loss compared to negative control.38,39,48,49 In addition, these dentifrices are effective in erosive-abrasive models.38 Similar results have been observed with tin-containing mouthrinse solutions, which have achieved a more-than 50% reduction in enamel wear.50,51 Like stannous products, titanium tetrafluoride solutions or varnishes form a protective layer when they react with mineral structure, reducing enamel hardness loss after the erosion process.52-54 Products containing proteins or chitosan have been shown to influence erosive wear. Jager et al reported the preventive effects of colostrum-containing toothpaste against erosion in a preclinical in situ study.55 Chitosan, a natural polysaccharide found in crustaceans, in combinations with tin has been used to create a protective layer on dental enamel, reducing structure loss and enhancing the action of the tin layer.40
In addition to fluoride products, phosphate-derived dentifrices containing sodium tri-meta phosphate interact with enamel to create a protective layer to reduce erosive wear. This type of product facilitates diffusion of calcium and fluoride ions56,57 and blocks acid diffusion, decreasing surface softening.
Also, magnesium-containing products, such as dentifrices (pH = 9.96), can be used to reduce erosive challenges.58 This is due to the presence of magnesium ions during demineralization and remineralization reactions, which changes crystallographic enamel structure because magnesium ions are incorporated in the surface layer of enamel, reducing the apatite crystallite size and increasing the hardness of the tooth.59 Furthermore, sodium bicarbonate solution has demonstrated efficacy in reducing tooth loss when used after the intrinsic erosive challenge has occurred, as it presents alkaline properties and buffer capacity, neutralizing oral cavity acids.60 Similarly, an antacid suspension containing sodium alginate, sodium bicarbonate, and calcium carbonate has shown to reduce enamel surface loss and, thus, may serve as a preventive product to address erosive challenge.61
Another over-the-counter product used to minimize the harmful effects of erosion is a dentifrice comprised of a formula consisting of particles constituted by hydroxyapatite and whose biomimetic characteristics present mineralizing and restorative action.62
Referral to Specialist
Referrals to specialists often are necessary for preventing dental erosion. Patients with GERD, for example, must be referred to a gastroenterologist for treatment. According to the global classification of GERD in the Montreal Criteria, the presence of erosion on lingual and palatal tooth surfaces is high in patients with GERD.13 The same is true in patients with anorexia-bulimia, where gastric acid contact is due to psychological factors.13 A referral of the patient for psychological or psychiatric treatment and nutritional counseling might be appropriate. Proper diagnosis and treatment by a physician and/or gastroenterologist may be needed to prevent further systemic complications in these patients.
Severe Cases
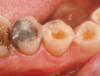
Due to the irreversible nature of dental erosion, cases may present in which the state of the tooth is such that excessive dentin has been exposed or the pulp has been severely compromised. In extreme cases, dental erosion can indirectly cause restoration failure by compromising the tooth/restoration interface or by weakening the surrounding tooth structure leading to restoration fracture. In Figure 13, the defective amalgam restoration demonstrates how the surrounding enamel is worn, leaving dentin exposed. Exposed dentin is at greater risk of erosion and demineralization if left untreated. These surfaces will then be susceptible to bacterial attack and recurrent caries. Proper management of erosive lesions can save the tooth from future caries risk.
Conclusions
Erosive mineral loss can have a significant impact on the function, shape, and esthetics of a tooth and cause hypersensitivity. This irreversible process can be prevented by identifying intrinsic and/or extrinsic sources of the condition. In many cases, tooth loss initiated by erosion is accentuated with abrasion and attrition.5 The clinical management of these sources requires an accurate diagnosis with a proper understanding of the etiology of the condition to help formulate a personalized preventive strategy. To address dental erosion, physicians and dentists need to know how to detect early signs through performing complete personal, medical, and dental histories and comprehensive intraoral examinations.
About the Author
Vanara F. Passos, DDS, PhD
Assistant Professor, Division of Operative Dentistry, Department of Restorative Dentistry, Federal University of Ceará, Fortaleza, Brazil
Mary A.S. Melo, DDS, PhD
Associate Professor, Division of Operative Dentistry, Department of General Dentistry, University of Maryland School of Dentistry, Baltimore, Maryland
Jennifer Park, DDS
Graduate, Division of Operative Dentistry, Department of General Dentistry, University of Maryland School of Dentistry, Baltimore, Maryland; Private Practice, Springfield, Virginia
Howard E. Strassler, DMD
Professor, Division of Operative Dentistry, Department of General Dentistry, University of Maryland School of Dentistry, Baltimore, Maryland
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Bartlett DW, Lussi A, West NX, et al. Prevalence of tooth wear on buccal and lingual surfaces and possible risk factors in young European Adults. J Dent. 2013;41(11):1007-1013.
2. Lussi A, Jaeggi T. Erosion-diagnosis and risk factors. Clin Oral Investig. 2008;12(suppl 1):S5-S13.
3. de Melo MA, Passos VF, Lima JP, et al. Carbohydrate-electrolyte drinks exhibit risks for human enamel surface loss. Restor Dent Endod. 2016;41 (4):246-254.
4. Ranjitkar S, Smales RJ, Kaidonis JA. Oral manifestations of gastroesophageal reflux disease. J Gastroenterol Hepatol. 2012;27(1):21-27.
5. Bishop K, Kelleher M, Briggs P, Joshi R. Wear now? An update on the etiology of tooth wear. Quintessence Int. 1997;28(5):305-313.
6. Petersen PE, Kwan S. The 7th WHO Global Conference on Health Promotion - towards integration of oral health (Nairobi, Kenya 2009). Community Dental Health. 2010;27(suppl 1):S129-S136.
7. Carvalho TS, Colon P, Ganss C, et al. Consensus Report of the European Federation of Conservative Dentistry: erosive tooth wear diagnosis and management. Swiss Dent J. 2016;126(4):342-346.
8. Larsen MJ. Erosion of the teeth. In: Fejerskov O, Kidd E, eds. Dental Caries: The Disease and Its Clinical Management. 2nd ed. Oxford, UK: Blackwell Munksgaard; 2008:233-247.
9. Ganss C, Lussi A. Diagnosis of erosive tooth wear. Monogr Oral Sci. 2014;25:22-31.
10. Sabandal MM, Schäfer E. Amelogenesis imperfecta: review of diagnostic findings and treatment concepts. Odontology. 2016;104(3):245-256.
11. Melo MA, Sousa R, Passos VF, et al. Erosion-preventive effect of pediatric fluoridated toothpaste on primary enamel. Int J Clin Pediatr Dent. 2016;9:(4)1-5.
12. Polland KE, Higgins F, Orchardson R. Salivary flow rate and pH during prolonged gum chewing in humans. J Oral Rehabil. 2003;30(9):861-865.
13. Pace F, Pallotta S, Tonini M, et al. Systematic review: gastro-oesophageal reflux disease and dental lesions. Aliment Pharmacol Ther. 2008;27(12):1179-1186.
14. Wegehaupt FJ, Lunghi N, Hogger VM, Attin T. Erosive potential of vitamin and vitamin+mineral effervescent tablets. Swiss Dent J. 2016;126(5):457-465.
15. Strużycka I, Rusyan E, Bogusławska-Kapała A. Epidemiological study of prevalence and risk factors for dental erosions among Polish young adults. Pol Merkur Lekarski. 2016;40(239):308-313.
16. Nankar M, Walimbe H, Ahmed Bijle MN, et al. Comparative evaluation of cariogenic and erosive potential of commonly prescribed pediatric liquid medicaments: an in vitro study. J Contemp Dent Pract. 2014;15(1):20-25.
17. de Melo MA, Passos VF, Lima JP, et al. Erosive potential of processed and fresh orange juice on human enamel. J Dent Child (Chic). 2015;82(1):10-15.
18. Søvik JB, Skudutyte-Rysstad R, Tveit AB, et al. Sour sweets and acidic beverage consumption are risk indicators for dental erosion. Caries Res. 2015;49(3):243-250.
19. Ehlen LA, Marshall TA, Qian F, et al. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008;28(5):299-303.
20. de Melo MA, Passos VF, Apolonio FM, et al. Restoring esthetics in eroded anterior teeth: a conservative multidisciplinary approach. Gen Dent. 2011;59(1):48-52.
21. Wang X, Lussi A. Assessment and management of dental erosion. Dent Clin North Am. 2010;54(3):565-578.
22. Reddy A, Norris DF, Momeni SS, et al. The pH of beverages in the United States. J Am Dent Assoc. 2016;147(4):255-263.
23. Wiegand A, Schwerzmann M, Sener B, et al. Impact of toothpaste slurry abrasivity and toothbrush filament stiffness on abrasion of eroded enamel - an in vitro study. Acta Odontol Scand. 2008;66(4):231-235.
24. Nakamura M, Kitasako Y, Nakashima S, et al. Impact of toothpaste on abrasion of sound and eroded enamel: an in vitro white light interferometer study. Am J Dent. 2015;28(5):268-272.
25. Bartlett D. A personal perspective and update on erosive tooth wear - 10 years on: Part 2 - restorative management. Br Dent J. 2016;221(4):167-171.
26. Dawes C, Boroditsky CL. Rapid and severe tooth erosion from swimming in an improperly chlorinated pool: case report. J Can Dent Assoc. 2008;74(4):359-361.
27. George R, Chell A, Chen B, et al. Dental erosion and dentinal sensitivity amongst professional wine tasters in South East Queensland, Australia. ScientificWorldJournal. 2014;16:516975.
28. West NX, Joiner A. Enamel mineral loss. J Dent. 2014;(42 suppl):S2-S11.
29. Lourenço M, Azevedo Á, Brandão I, Gomes PS. Orofacial manifestations in outpatients with anorexia nervosa and bulimia nervosa focusing on the vomiting behavior. Clin Oral Investig. 2018;22(5):1915-1922.
30. Bartlett D, Ganss C, Lussi A. Basic Erosive Wear Examination (BEWE): a new scoring system for scientific and clinical needs. Clin Oral Investig. 2008;12(suppl 1):S65-S68.
31. Olley RC, Wilson R, Bartlett D, Moazzez R. Validation of the Basic Erosive Wear Examination. Caries Res. 2014;48(1):51-56.
32. Kilpatrick N, Mahoney EK. Dental erosion: part 2. The management of dental erosion. N Z Dent J. 2004;100(2):42-47.
33. Honório HM, Rios D, Júnior ES, et al. Effect of acidic challenge preceded by food consumption on enamel erosion. Eur J Dent. 2010;4(4):412-417.
34. de Alencar CR, Magalhães AC, de Andrade Moreira Machado MA, et al. In situ effect of a commercial CPP-ACP chewing gum on the human enamel initial erosion. J Dent. 2014;42(11):1502-1507.
35. Passos VF, de Melo MA, Vasconcelos AA, et al. Comparison of methods for quantifying dental wear caused by erosion and abrasion. Microsc Res Tech. 2013;76(2):178-183.
36. Li X, Wang J, Joiner A, Chang J. The remineralisation of enamel: a review of the literature. J Dent. 2014;42(suppl 1):S12-S20.
37. Hellwig E, Lussi A. Oral hygiene products, medications and drugs-hidden aetiological factors for dental erosion. Monogr Oral Sci. 2014;25:155-162.
38. Passos VF, de Vasconcellos AA, Pequeno JH, et al. Effect of commercial fluoride dentifrices against hydrochloric acid in an erosion-abrasion model. Clin Oral Investig. 2015;19(1):71-76.
39. Bellamy PG, Harris R, Date RF, et al. In situ clinical evaluation of a stabilised, stannous fluoride dentifrice. Int Dent J. 2014;64(suppl 1):43-50.
40. Passos VF, Melo MA, Silva FF, et al. Effects of diode laser therapy and stannous fluoride on dentin resistance under different erosive acid attacks. Photomed Laser Surg. 2014;32(3):146-151.
41. de Melo MA, Silva FF, Passos VF, et al. Investigation on light-assisted preventive effects on dentin erosion. Photon Lasers Med.2013;2(3):209-216.
42. Zhao X, He T, He Y, et al. A randomized clinical trial to measure the erosion protection benefits of a novel stabilized stannous fluoride dentifrice versus a control dentifrice. J Clin Dent. 2017;28(spec no B):B17-B20.
43. Magalhães AC, Wiegand A, Rios D, et al. Fluoride in dental erosion. Monogr Oral Sci. 2011;22:158-170.
44. Bakry AS, Marghalani HY, Amin OA, Tagami J. The effect of a bioglass paste on enamel exposed to erosive challenge. J Dent. 2014;42(11):1458-1463.
45. Sun Y, Li X, Deng Y, et al. Mode of action studies on the formation of enamel minerals from a novel toothpaste containing calcium silicate and sodium phosphate salts. J Dent. 2014;42(suppl 1):S30-S38.
46. Joiner A, Schäfer F, Naeeni MM, et al. Remineralisation effect of a dual-phase calcium silicate/phosphate gel combined with calcium silicate/phosphate toothpaste on acid-challenged enamel in situ. J Dent. 2014;42(suppl 1):S53-S59.
47. Parker AS, Patel AN, Al Botros R, et al. Measurement of the efficacy of calcium silicate for the protection and repair of dental enamel. J Dent. 2014;42(suppl 1):S21-S29.
48. Huysmans MC, Jager DH, Ruben JL, et al. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45(6):518-523.
49. Hove LH, Stenhagen KR, Mulic A, et al. May caries-preventive fluoride regimes have an effect on dental erosive wear? An in situ study. Acta Odontol Scand. 2015;73(2):114-120.
50. Schlueter N, Klimek J, Ganss C. Efficacy of an experimental tin-F-containing solution in erosive tissue loss in enamel and dentine in situ. Caries Res. 2009;43(6):415-421.
51. Schlueter N, Klimek J, Ganss C. Efficacy of tin-containing solutions on erosive mineral loss in enamel and dentine in situ. Clin Oral Investig. 2011;15(3):361-367.
52. Levy FM, Rios D, Buzalaf MA, Magalhães AC. Efficacy of TiF4 and NaF varnish and solution: a randomized in situ study on enamel erosive-abrasive wear. Clin Oral Investig. 2014;18(4):1097-1102.
53. Magalhães AC, Rios D, Honório HM, et al. Effect of 4% titanium tetrafluoride solution on the erosion of permanent and deciduous human enamel: an in situ/ex vivo study. J Appl Oral Sci. 2009;17(1):56-60.
54. Stenhagen KR, Hove LH, Holme B, Tveit AB. The effect of daily fluoride mouth rinsing on enamel erosive/abrasive wear in situ. Caries Res. 2013;47(1):2-8.
55. Jager DH, Vissink A, Timmer CJ, et al. Reduction of erosion by protein-containing toothpastes. Caries Res. 2013;47(2):135-140.
56. Cruz NV, Pessan JP, Manarelli MM, et al. In vitro effect of low-fluoride toothpastes containing sodium trimetaphosphate on enamel erosion. Arch Oral Biol. 2015;60(9):1231-1236.
57. Takeshita EM, Exterkate RA, Delbem AC, ten Cate JM. Evaluation of different fluoride concentrations supplemented with trimetaphosphate on enamel de- and remineralization in vitro. Caries Res. 2011;45(5):494-497.
58. Passos VF, Rodrigues LK, Santiago SL. The effect of magnesium hydroxide-containing dentifrice using an extrinsic and intrinsic erosion cycling model. Arch Oral Biol. 2018;86:46-50.
59. Abdallah MN, Eimar H, Bassett DC, et al. Diagenesis-inspired reaction of magnesium ions with surface enamel mineral modifies properties of human teeth. Acta Biomater. 2016;37:174-183.
60. Messias DC, Turssi CP, Hara AT, Serra MC. Sodium bicarbonate solution as an anti-erosive agent against simulated endogenous erosion. Eur J Oral Sci. 2010;118(4):385-388.
61. Alves Mdo S, Mantilla TF, Bridi EC, et al. Rinsing with antacid suspension reduces hydrochloric acid-induced erosion. Arch Oral Biol. 2016;61:66-70.
62. Poggio C, Gulino C, Mirando M, et al. Preventive effects of different protective agents on dentin erosion: an in vitro investigation. J Clin Exp Dent. 2017;9(1):e7-e12.