You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Currently, tooth loss is often accompanied by either immediate implant insertion or alveolar ridge preservation techniques. Both of these procedures are geared towards tooth replacement prior to the removal of the tooth. Recent studies have demonstrated that implant placement into an extraction socket will not prevent alveolar ridge resorption as was once thought.1,2 Immediate implant placement is combined with bone grafting to prevent bone atrophy around the implant, which could compromise the level of osseointegration and esthetic outcomes. Socket-preservation procedures are geared towards preserving the horizontal and vertical dimensions of the ridge to a large degree, facilitating favorable implant placement.
When a tooth or multiple teeth are already lost, either from periodontitis, caries, endodontic involvement, or trauma, the opportunity to preserve bone is no longer a possibility. The subsequent bone loss that follows extraction can be quite dramatic, and often occurs in the first few months after extraction.3 This resultant bone loss can result in the center of the alveolar ridge being “relocated” to a more lingual/palatal position.4,5 While it is possible for implant placement to be performed in these sites, the occlusal forces directed upon the restorations would not be distributed along the long axis of the endosseous fixture, increasing the risks of biomechanical complications. Implants placed lingually/palatally may also necessitate restorations with buccal ledges for esthetic purposes, which can compromise plaque removal. It would therefore seem logical that reconstructive surgery aimed at repositioning the center of the alveolar ridge buccally could obviate or minimize some of these issues.
One of the most frequently used procedures for this type of reconstruction is the autogenous block graft. Misch6 demonstrated favorable results using mandibular bone for this purpose. This procedure can result in significant gain in bone volume for implant placement. There are, however, disadvantages to this approach. Morbidity associated with harvesting autogenous bone blocks includes pain, swelling, decreased innervation, and even tooth devitalization in certain instances.7 Another disadvantage to block grafting is the predictable resorption that occurs at the recipient site. Cordaro8 demonstrated approximately 25% horizontal and more than 40% vertical resorption of autogenous block grafts. In a follow-up article, Cordaro9 demonstrated significantly less resorption of autogenous block grafts when combined with a particulate bone graft of bovine origin and a porcine GBR membrane. The authors also noted more complications were related to the sites augmented with exogenous materials compared to block grafts alone.
In an animal study, Kim et al10 demonstrated that combining single and even double layers of collagen barrier membranes with block grafts can significantly reduce the amount of resorption. Interestingly, Roccuzzo et al11 demonstrated that when an autogenous block graft was combined with a titanium mesh, significantly less resorption of the block occurred. From the literature, it can be concluded that autogenous block grafts are effective in terms of gaining 3-D bone volume to facilitate dental implant placement. It cannot be ignored, however, that as in most surgical modalities, shortcomings exist. Alternative means of gaining bone regrowth have been attempted.
One of the most frequently employed methods of bone reconstruction is the use of a rigid mesh. Most commercially available meshes are composed of titanium. The use of titanium mesh for alveolar ridge augmentation is not a novel concept. Historically, Boyne et al12 demonstrated that combining titanium mesh with autogenous bone harvested from the iliac crest is predictable in reconstructing severely atrophic maxillary edentulous ridges. Von Arx and Kurt13 demonstrated the effectiveness of titanium mesh in repairing osseous defects associated with early implant placement. The mesh was used for containment of autogenous bone over dehiscence-type defects, and the authors reported approximately 93% defect resolution.
Although the space-maintaining properties of titanium mesh is clinically proven to be effective, there are disadvantages to this modality, including early exposure. In a recent study, Miyamoto et al14 noted premature exposure to have occurred in 36% of the cases on which they reported. Although early exposure was not an absolute cause for its early removal, they reported on the need to remove the titanium mesh prematurely in 8% of the cases. Partial bone resorption with minor infection was noted in 10% of these situations. In a case series of five patients, Misch15 demonstrated no premature mesh exposures when combining titanium mesh with a composite graft composed of recombinant human bone morphogenetic protein-2/absorbable collagen sponge (rhBMP-2/ACS) and freeze-dried bone allograft (FDBA). The present author16 has speculated that the wound-healing properties of rhBMP-2 may have contributed to accelerated soft-tissue healing in that case series.
Nevertheless, the major drawback to titanium mesh for ridge reconstruction is the necessity of its removal. Often the scope of re-entry surgery to remove a titanium mesh is as invasive as the procedure to augment the ridge. This mesh is usually well incorporated into the regenerated site and covered with a dense connective tissue, referred to by Boyne as a “pseudoperiosteum.” This step may add significant time to the procedure and subsequently increase postoperative morbidity. The possibility of performing a computer-guided, transmucosal implant surgery is also eliminated, because a mucoperiosteal flap must be reflected to remove the mesh and fixation screws or tacks. In a previous article, the author16 presented a technique using a resorbable mesh (RapidSorb, Synthes, www.synthes.com) for augmentation of extraction sockets lacking facial and or lingual cortices. The mesh, composed of a copolymer of polylactide (85%) and polyglycolide (15%), was combined with either mineralized allograft bone (FDBA) or rhBMP-2/ACS.
The purpose of this article is to demonstrate the use of the same bioresorbable mesh with a composite graft of rhBMP-2/ACS + FDBA for reconstruction of atrophic alveolar ridges, for which implant insertion requires pre-placement bone reconstruction.
Method and Materials
The following cases demonstrate the use of the above-described technique of horizontal ridge augmentation with a bioresorbable mesh and the composite osteoinductive/osteoconductive bone graft.
Case 1
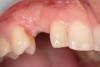
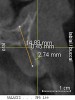
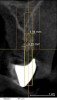
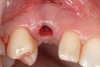
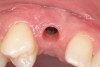
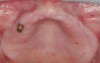
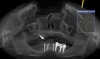
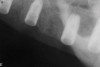
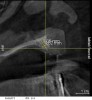
A 26-year-old woman presented with a history of trauma combined with an impacted maxillary right canine (No. 6), which was extracted in early childhood. Following two courses of orthodontic therapy—one in her early teens and the second in early adulthood—the area had reportedly been grafted approximately 9 months prior to her initial presentation to the author’s private periodontal practice. Clinically, the edentulous site appeared healthy, with significant keratinized mucosa in the canine position (Figure 1). A cone-beam CT scan revealed severe horizontal bone deficiency in the proposed implant position (Figure 2). Adequate bone height for implant placement was evident; however, the thinnest portion of the ridge measured approximately 0.45 mm in width. It was proposed that an augmentation be performed to facilitate implant placement, which the patient accepted.
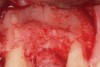
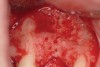
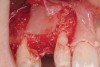
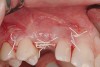
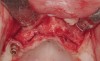
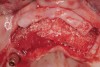
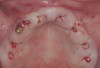
Following administration of local anesthesia (2.4 cc of 4% articaine with epinephrine 1/100,000 [Septocaine®, Septodont, www.septodontusa.com]) and premedication with amoxicillin (1,000 mg 1 hour prior to surgery and beginning a course of methylprednisolone [Medrol Dosepak, Pfizer, www.pfizer.com]), a full-thickness flap was reflected with buccal-releasing incisions on the proximal surfaces of the adjacent teeth. The severe ridge deficiency is evident in Figure 3. After removal of any soft-tissue adhesions to the bone surface with ultrasonic and manual instrumentation, numerous cortical penetrations with a #2 round carbide bur were performed to increase vascularity to graft (Figure 4). A composite graft composed of 0.8 mg of rhBMP-2/ACS (INFUSE® bone graft, Medtronic Inc., www.medtronic.com) + 0.5 cc of FDBA (OraGraft®, LifeNet Health, www.accesslifenethealth.org) was homogenously mixed extraorally and placed over the bony defect. A template was used to “size” the resorbable mesh; then the mesh was trimmed and warmed in a water bath at 70 degrees Centigrade to make the material temporarily malleable to shape, according to the metal temple, for close adaptation to the defect over the bone graft that was lying passively beneath the palatal flap. Two resorbable screws, consisting of the same polymer, were secured at the apical corners of the mesh buccally, respecting a safe distance from the apices of the adjacent teeth. A small portion of the rhBMP-2-seeded ACS was then passively placed over the buccal portion of the mesh for containment of FDBA particles (Figure 5). A periosteal-releasing incision was done apically, and a tension-free primary closure was achieved (Figure 6). Antibiotics were continued for 10 days, and the patient was advised to avoid mastication or tooth brushing in the maxillary right quadrant until sutures were removed in about 9 days. A tooth-borne Essex retainer was worn as a provisional restoration, avoiding any contact with the operated site.
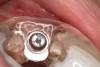
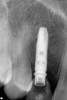
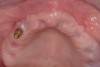
Approximately 4 months after grafting, the patient returned for clinical and 3-D radiographic evaluation. The thinnest area of ridge width preoperatively was remeasured in approximately the same location, demonstrating a gain of about 4 mm (Figure 7). The treatment plan was to place a 3.5-mm x 13-mm implant with a computer-generated guide (SiCat, Sirona Dental, www.sironausa.com), eliminating an additional open surgical procedure. Planning included initial osteotomy preparation with single-use drills combined with a localized ridge expansion using narrow, tapered osteotomes. This was performed approximately 5 months after the augmentation procedure, achieving primary stability of the implant, facilitating a transmucosal healing approach (Figure 8 and Figure 9). Following each step of osteotomy preparation, a probe was inserted along the walls of the site to confirm the integrity of the buccal and palatal walls prior to implant insertion.
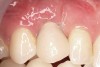
Ten weeks after implant insertion, the patient presented to begin soft-tissue contouring via a fixed, provisional crown (Figure 10). Deliberate under-contouring of the cervical portion of the temporary restoration was performed to avoid unwanted mucosal recession and possible esthetic complications (Figure 11 and Figure 12).
The author notes that, unfortunately, this patient has not returned after being referred to her general dentist for definitive restorative therapy.
Case 2
A 54-year-old woman who had been edentulous for more than 10 years presented to the author’s practice. She had previously undergone implant therapy in her mandibular left posterior sextant, and recently had a “mini”-implant procedure in the remainder of her mandibular arch, supporting a removable prosthesis. She also had several mini implants placed in her maxilla and an overdenture fabricated. Three of the four implants did not achieve osseointegration and were removed by her dentist. The mini implant in the maxillary right first bicuspid position served as a retentive anchor for a full denture (Figure 13 and Figure 14).
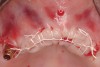
Clinical and radiographic evaluation revealed significant alveolar ridge resorption and maxillary sinus pneumatization. The patient was informed that to achieve her goal of wearing a fixed prosthesis, she would require bilateral sinus grafts and anterior ridge augmentation, which she agreed to undergo. Following augmentation of her right and left maxillary sinuses, she presented for reconstruction of her severely atrophic anterior region. Following reflection of a full-thickness flap, severe bone loss was evident, especially in the right canine–lateral incisor region (Figure 15). Following decortication of the buccal cortex with a #2 round bur, a composite graft consisting of 0.8 mg of rhBMP-2/ACS + 0.7 cc of FDBA was adapted to the facial surface of the ridge from the maxillary right to the left canine regions. A bioresorbable PLGA mesh was then contoured and affixed with two PLGA screws 4 mm in length and 1.5 mm in diameter. Additional particulate FDBA graft was then placed under the mesh to obturate the entire space between the bony surface of the ridge and the mesh (Figure 16). A non-cross-linked collagen tape (CollaTape®, Zimmer Dental, www.zimmerdental.com) was applied over the mesh for containment of the particulate bone graft, followed by periosteal-releasing incisions and tension-free primary closure with monofilament polytetrafluoroethylene (PTFE) sutures (Figure 17).
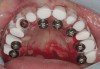
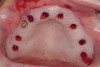
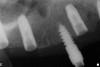
The patient was scanned with a cone beam CT scan while wearing a radiopaque scanning appliance based on her new treatment denture. Horizontal bone augmentation was confirmed radiographically, and both grafted sinuses resulted in satisfactory bone quantity for implant placement. Vertical augmentation was not attempted because of the patient’s unwillingness to forego her removable prosthesis for any period of time. Therefore, shorter implants were treatment-planned, resulting in the placement of eight implants, rather than fewer—such as six—implants, to support a full-arch fixed prosthesis. Because adequate bone and keratinized mucosa were present, a flapless, computer-guided implant insertion was performed (Figure 18). After removal of the surgical guide, placement of all eight implants could be inspected (Figure 19). Post-placement periapical radiographs are shown in Figure 20 and Figure 21.
The surgery was initiated with eight mucoplasties using a disposable tissue punch. The autogenous, epithelialized grafts were maintained in aseptic conditions throughout surgery. After gentle irrigation of the implant sites with sterile saline, these eight tissue grafts were re-placed using resorbable sutures to prevent contamination of the implant sites and provide protection from the overlying treatment denture (Figure 22). The transitional denture was relieved, and a resilient reline was performed at the conclusion of surgery. The patient started a 10-day course of amoxicillin 500 mg three times daily, 1 hour prior to surgery, as well as a 6-day tapering dose of methylprednisolone 4 mg the morning of surgery. She was also prescribed an NSAID, etodolac 400 mg to be taken every 8 hours for 3 days, and a chlorhexidine gluconate rinse twice daily. She was advised to only wear the denture when absolutely necessary, and to always remove it before bedtime.
At approximately 3 weeks after implant surgery, four of the eight free-gingival “plugs” appeared incorporated over the underlying implants, and no communication with the cover screws was evident (Figure 23). A postoperative CT scan demonstrated horizontal bone regeneration compared to the preoperative situations, as demonstrated in the region of tooth No. 9 (Figure 24 and Figure 25).
The author notes that this patient put restorative treatment on hold due to financial reasons.
Discussion
The cases presented in this article represent several novel concepts in alveolar ridge reconstruction. First, the combination of rhBMP-2 and a mineralized allograft can serve as an osteoinductive and osteoconductive bone graft. In a case series, Misch17 demonstrated excellent bone maintenance and osseointegration of dental implants placed into sites of previously grafted extraction sockets with this type of bone graft. An advantage of grafting extraction sockets is that the surgeon can to some degree, though not completely, prevent the predictable resorption of alveolar bone following tooth removal.18 Use of a demineralized bone allograft, which is osteoconductive and potentially osteoinductive, was not done in this series, mainly because of the unpredictability of the allograft to serve an osteoinductive role.19 Also, the rapid resorption of demineralized bone grafts may result in suboptimal bone quality at the time of implant insertion. The mineralized bone (FDBA) used here has a slower substitution rate and better space-maintenance properties than demineralized bone. The inductive property of DFDBA was not required in these cases because the rhBMP-2 component of the graft is capable of osteoblastic differentiation.20,21 The cases presented in this article demonstrate the horizontal increase of alveolar ridge dimensions of approximately 1 mm to 4 mm. These dimensions facilitate implant-placement capable of long-term physiologic osseointegration.22
Historically, animal models have demonstrated the ability of rhBMP-2 to induce de novo bone formation in sinus grafts.23,24 The combination of rhBMP-2 with matrices other than the ACS packaged commercially with the protein has also proven effective in bone regeneration in animal models.25 Practically, the expense of commercially available rhBMP-2 has prevented its more routine use in practice. As the dosage/quantity of the protein increases, the cost does as well. Using a smaller dose (0.8 mg), as in this case series, and combining it with a reasonably cost-effective particulate bone graft such as FDBA, can expand the overall graft volume for larger defects and provide a proven osteoconductive component to an inductive graft incapable of space maintenance. Certainly in contemporary practice, economics play a role in selection of biomaterials used for reconstructive procedures. Other growth factors, in particular recombinant human platelet-derived growth factor-BB (rhPDGF-BB) (ie, Gem 21S®, Osteohealth, www.osteohealth.com), have received extensive attention. Investigators have demonstrated in animals and humans the combination of xenograft blocks or barrier membranes and rh-PDGF-BB being efficacious for alveolar bone reconstruction.26,27 It is noteworthy, however, that currently the only commercially available osteoinductive growth factor is rhBMP-2. Other bone morphogenetic proteins, such as rhBMP-7, appear to be approaching implementation into private practice; however, growth factors such as rhPDGF-BB are chemotactic, mitogenic, and angiogenic, but not osteoinductive. Much of the literature supporting its use is focused on its successful utilization in periodontal regeneration.28,29 Periodontal regeneration differs from alveolar ridge augmentation in that regeneration of lost cementum, alveolar bone, and periodontal ligament (PDL) fibers are required for true periodontal regeneration. Ridge augmentation facilitating dental implant placement requires the reconstruction of alveolar bone capable of achieving osseointegration. The etiology of periodontal attachment loss is usually of microbial origin. The presence of plaque and calculus, combined with the local host immune response, is responsible for such bone loss. Atrophic ridges result after tooth loss. The embryologic function of alveolar bone to support teeth disappears after extraction, resulting in 3-dimensional bone loss. If the goal of ridge augmentation is the reconstruction of bone, therapies should be aimed at predictable results. The need for space can be combined with signaling molecules, such as rhBMP-2, capable of inducing mesenchymal stem cells to differentiate into osteoblasts.
The addition of an osteoconductive yet resorbable particulate serves several purposes. First, the FDBA particles support bony ingrowth or scaffolding. Their addition to rhBMP-2/ACS also expands the graft volume, limiting the overall dose of rhBMP-2 required to obturate the space between the mesh and the walls of the defect. Though the soft-tissue edema often associated with rhBMP-2 is transient, it has been shown to be dose-dependent to some degree.30 The composite graft utilized in the technique presented here exploits the osteoinductivity of rhBMP-2 and the osteoconductivity of FDBA. The requirement of space maintenance is fulfilled by the resorbable mesh.
The resorbable mesh has several advantages over titanium mesh. First, the obvious elimination of a removal surgery decreases patient morbidity. This in itself must be viewed as a benefit over its titanium predecessor. The lack of metal also makes it easier to read radiographs, including CT scans, without artifacts created by submucosal metal componentry. This also creates the opportunity for computer-guided implant placement without reflection of a mucoperiosteal flap. Not only does this decrease morbidity for the patient, but it may increase case acceptance because one large surgical procedure can be minimized or avoided. Another advantage is that if a portion of the mesh becomes exposed to the oral cavity, it can be trimmed and removed and does not necessitate complete mesh removal, simplifying maintenance.
Conclusion
The evolution of bioresorbable scaffolds and bioactive grafting materials continues to minimize the need to harvest autogenous bone for alveolar ridge reconstruction. The combination of these materials exploits the advantages of each individual material, demonstrating a possible “synergy” of newer-generation techniques for the surgeon to reconstruct severely atrophic ridges, facilitating implant placement.
It should be stated that this follow-up paper demonstrates the author’s evolving experience with a technique of alveolar ridge augmentation. These “off-label” uses of such biomaterials has resulted in predictable clinical results, yet lacks controlled studies. Perhaps as interest in this modality expands, such studies will be performed to further support its widespread use in such indications.
References
1. Araújo MG, Sukekava F, Wennström JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32(6):645-652.
2. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820-828.
3. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
4. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.
5. Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust Dent J. 1969;14(4):241-244.
6. Misch CM, Misch CE. Autogenous mandibular bone grafts for reconstruction of ridge deficiencies prior to implant placement [abstract]. Int J Oral Maxillofac Implants. 1993;(8);117.
7. Misch CM, Mish CE. The repair of localized severe ridge defects for implant placement using mandibular bone grafts. Implant Dent. 1995;4(4):261-267.
8. Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002;13(1):103-111.
9. Cordaro L, Torsello F, Morcavallo S, di Torresanto VM. Effect of bovine bone and collagen membranes on healing of mandibular bone blocks: a prospective randomized controlled study. Clin Oral Impl Res. 2011;22(10):1145-1150.
10. Kim SH, Kim DY, Kim KH, et al. The efficacy of a double-layer collagen membrane technique for overlaying block grafts in a rabbit calvarium model. Clin Oral Implants Res. 2009;20(10):1124-1132.
11. Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18(3):286-294.
12. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43(2):87-91.
13. von Arx T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh: a prospective clinical study with 20 implants. Clin Oral Implants Res. 1999;10(1):24-33.
14. Miyamoto I, Funaki K, Yamauchi K, et al. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant Dent Relat Res. 2012;14(2):304-311.
15. Misch CM. Bone augmentation of the atrophic posterior mandible for dental implants using rhBMP-2 and titanium mesh: clinical technique and early results. Int J Periodontics Restorative Dent. 2011;31(6):581-589.
16. Levin BP. Horizontal alveolar ridge augmentation: the importance of space maintenance. Compend Contin Educ Dent. 2011;32(8):12-21.
17. Misch CM. The use of recombinant human bone morphogenetic protein-2 for the repair of extraction socket defects: a technical modification and case series report. Int J Oral Maxillofac Implants. 2010;25(6):1246-1252.
18. Nevins M, Camelo M, De Paoli S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19-29.
19. Schwartz Z, Somers A, Mellonig JT, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 1998;69(4):470-478.
20. Mahy PR, Urist MR. Experimental heterotopic bone formation induced by bone morphogenetic protein and recombinant human interleukin-1B. Clin Orthop Relat Res. 1988;237:236-244.
21. Urist MR, Mikulshi AJ, Nakagawa M, Yen K. A bone matrix calcification-initiator noncollagenous protein. Am J Physiol. 1977;232(3):C115-C127.
22. Spray JR, Black CG, Morris HF, Ochi S. The influence of bone thickness on facial marginal bone response: stage 1 placement through stage 2 uncovering. Ann Periodontol. 2000;5(1):119-128.
23. Nevins M, Kirker-Head C, Nevins M, et al. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996;16(1):8-19.
24. Wada K, Niimi A, Watanabe K, et al. Maxillary sinus floor augmentation in rabbits: A comparative study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001;21(3):253-263.
25. Jung RE, Weber FE, Thoma DS, et al. Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydroxyapatite/tricalciumphosphate. Clin Oral Implants Res. 2008;19(2):188-195.
26. Nevins M, Al Hezaimi K, Schupbach P, et al. Vertical ridge augmentation using an equine bone and collagen block infused with recombinant human platelet-derived growth factor-BB: a randomized single-masked histologic study in non-human primates. J Periodontol. 2012;83(7):878-884.
27. Simion M, Rocchietta I, Monforte M, Maschera E. Three-dimensional alveolar bone reconstruction with a combination of recombinant human platelet-derived growth factor BB and guided bone regeneration: a case report. Int J Periodontics Restorative Dent. 2008;28(3):239-243.
28. Nevins M, Camelo M, Nevins ML, et al. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282-1292.
29. Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213-225.
30. Bae HW, Strenge KB, Ashraf N, et al. Transient soft-tissue edema associated with implantation of increasing doses of rhBMP-2 on an absorbable collagen sponge in an ectopic rat model. J Bone Joint Surg Am. 2012;94(20):1845-1852.
Related Content:
This article is a follow-up to: Levin BP. Horizontal alveolar ridge augmentation: The importance of space maintenance. Compend Contin Educ Dent. 2011;32(8):12-21. Available from CDEWorld at dentalaegis.com/go/cced338
About the Author
Barry P. Levin, DMD
Private Practice
Elkins Park, Pennsylvania