You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The ADAA has an obligation to disseminate knowledge in the field of dentistry. Sponsorship of a continuing education program by the ADAA does not necessarily imply endorsement of a particular philosophy, product, or technique.
Evidence supports the belief that improved hand hygiene can reduce health care associated infection rates. Failure to perform recommended hand hygiene is considered one of the leading causes of health care associated infections. Unfortunately, frequent and repeated use of hand hygiene products, particularly soaps and other detergents, are a primary cause of chronic irritant contact dermatitis among healthcare workers.1 Discomfort due to irritation can interfere with adherence to recommended hand hygiene practices. Consequently, it is important to have different types of hand hygiene products and gloves available in the dental office.
History of Hand Hygiene
Hand washing was not always considered important. According to the 2002 Centers for Disease Control (CDC) Guideline for Hand Hygiene in Health-Care Settings, the concept of cleansing hands with an antiseptic agent probably emerged in the early 19th century. As early as 1822, the French pharmacist, Labarraque, demonstrated that solutions containing chlorides of lime or soda could eradicate the foul odors associated with human corpses and that such solutions could be used as disinfectants and antiseptics. In a paper published in 1825, Labarraque stated that physicians and other persons attending patients with contagious diseases would benefit from moistening their hands with a liquid chloride solution.1
In 1846, Ignaz Semmelweis, a Hungarian physician, observed that women whose babies were delivered by students and physicians in the First Clinic at the General Hospital of Vienna consistently had a higher mortality rate than those whose babies were delivered by mid-wives in the Second Clinic.1
Dr. Semmelweis noted that students and physicians who went directly from the autopsy suite to the obstetrics ward had a disagreeable odor on their hands despite washing their hands with soap and water. He claimed that the reason so many women in the clinic died from puerperal fever was due to the "cadaverous particles" transmitted to the obstetrics ward via the hands of students and physicians. He insisted that students and physicians clean their hands with a chlorine solution between each patient in the clinic. The maternal mortality rate in the First Clinic subsequently dropped dramatically and remained low for years. This intervention by Dr. Semmelweis represents the first evidence indicating that cleansing heavily contaminated hands with an antiseptic agent between patient contacts may reduce health care associated transmission of contagious diseases more effectively than hand washing with plain soap and water.1 Because of his contributions, Dr Semmelweis dubbed the "Father of Modern Infection Control".
Hand Hygiene Guidelines in the United States
In 1961, the Public Health Service produced the first training film on hand washing techniques directing healthcare workers (HCW) to wash their hands with soap and water for 1-2 minutes before and after patient contact. (The term "soap" is used as a lay term to reference "detergents" in this course.) These first guidelines were modified as new research studies were conducted.
In 1975 and again in 1985, the Centers for Disease Control (CDC) issued written guidelines on hand washing practices in hospitals. The guidelines recommended hand washing with non-antimicrobial soap between the majority of patient contacts and washing with antimicrobial soap before performing invasive procedures or caring for high risk patients. Use of waterless antiseptic agents (e.g., alcohol-based solutions) were recommended only in situations where sinks were not available.1
The most current CDC Guideline for Hand Hygiene in Health-Care Settings was issued in October 2002 and is still applicable for today's health care settings. The guideline states that improved hand hygiene practices can reduce transmission of pathogenic microorganisms to patients and HCWs in health care settings. This guideline was created for HCWs in hospital settings, however, it contains information relevant to the dental team as well.
Types of Hand Bacteria
The skin on hands is colonized with bacteria. These bacteria are divided into two categories; transient flora and resident flora. Transient (come and go) flora are considered the contaminating flora, and most likely to be associated with infection. Transient microorganisms are acquired during the exposure to any contaminated surface. Transient flora is easily removed by hand washing as it stays on the outer layers of the skin. Resident flora colonize within the deeper layers of the skin and are considered to be permanent. They cannot be totally removed, even with a surgical scrub. Resident flora is less likely to be associated with infection.8
Evidence of Transmission of Pathogens on Hands
Similar to the "chain of infection", transmission of health care-associated pathogens via the hands of HCWs requires the following sequence of events:
• Organisms present on the patient's skin, or those that have been shed onto inanimate objects in close proximity to the patient, must be transferred to the hands of the HCW;
• these organisms must be capable of surviving for at least several minutes on the hands of the HCW;
• hand washing or hand antisepsis techniques by the HCW must be inadequate or omitted entirely, or the agent used for hand hygiene must be inappropriate; and
• the contaminated hands of the HCW must come in direct contact with another patient, or with an inanimate object that will come into direct contact with the patient.1
Health Care-Associated Infections (HAIs) are infections that people acquire while they are receiving treatment for another condition in a health care setting. HAIs can be acquired anywhere health care is delivered. HAIs may be caused by any infectious agent, including bacteria, fungi, and viruses, as well as other less common types of pathogens.
These infections are associated with a variety of risk factors, including:
• Use of indwelling medical devices such as bloodstream, endotracheal, and urinary catheters
• Surgical procedures
• Injections
• Contamination of the health care environment
• Transmission of communicable diseases between patients and healthcare workers
• Overuse or improper use of antibiotics
• Magnitude of the Problem10
HAIs are a significant cause of morbidity and mortality. At any given time, about 1 in every 20 inpatients has an infection related to hospital care. These infections cost the U.S. health care system billions of dollars each year and lead to the loss of tens of thousands of lives. In addition, HAIs can have devastating emotional, financial and medical consequences.
A majority of hospital-acquired HAIs include:
• Urinary tract infections
• Surgical site infections
• Bloodstream infections
• Pneumonia10
In 2005, approximately 18,650 persons died after contracting a Methicillin-resistant Staphylococcus aureus (MRSA) infection during a hospital stay. In 2011, the CDC estimates over 80,000 MRSA infections and over 11,000 deaths.9 MRSA is the term used to describe a number of strains of the bacteria Staphylococcus aureus. These strains are resistant to a number of antibiotics including methicillin, oxacillin, clindamycin, vancomycin, and other more common antibiotics. S. aureus, often referred to as "staph," are bacteria commonly carried on the skin or in the noses of healthy people. Approximately 25% to 30% of the population colonizes staph bacteria in their noses. Although, staph can cause an infection,2 most staph are colonized - a term used in the medical profession that means bacteria are present but are not causing an infection.
According to the CDC, staph infections, including MRSA, occur most frequently among persons in hospitals and health care facilities, such as nursing homes and dialysis centers, who may have weakened immune systems. These types of infections are called Health Care Associated MRSA (HA-MRSA) infections. Patients who already have a MRSA infection, or who carry the bacteria on their bodies but do not have symptoms, are the most common sources of transmission in a health care facility. The main mode of transmission to other patients is through human hands, especially HCW's hands. Hands may become contaminated with MRSA bacteria by contact with infected or colonized patients. If appropriate hand hygiene such as washing with soap and water or using an alcohol-based hand sanitizer is not performed, the bacteria can be spread when the HCW touches other patients or surfaces (chain of infection sequence).2
MRSA and the Dental Office
Aerosols containing microbes from the oral cavity of the patient are created when using high-speed rotating instruments in restorative dentistry. How far these aerosols spread and what level of contamination they cause has become a growing concern as the number of patients with oro-nasal MRSA colonization increases. Two recent studies were conducted to determine if aerosols and spatter generated during patient treatment could spread MRSA.3,4
One study was conducted to determine how far airborne bacteria spread during dental treatment and the level of contamination that accumulated in the treatment room. Fallout samples were collected on blood agar plates placed in several locations of the treatment room. The plates were placed approximately 1½ to 6½ feet from the patient's mouth.
The results showed significant contamination at all distances sampled when high-speed instruments were used. Contamination was less intense during periodontal surgery and orthodontic treatment where high-speed rotating and ultrasonic instruments were not used. Gram-positive cocci, namely viridans streptococci and staphylococci, were the most common findings. The area that becomes contaminated during dental procedures is far larger than previously thought. These results emphasize the need for developing new means for preventing microbial aerosols in dentistry and protection of all items stored temporarily on work surfaces.3
In a second study,4 a survey of MRSA contamination on the surfaces of the dental treatment room, including the air-water syringe and the patient chair, was completed. This survey included an analysis of MRSA transmission via the dental treatment room between patients. Results showed that nosocomial infections or colonization of MRSA occurred in eight out of 140 consecutive patients who had no evidence of MRSA at admission. It was confirmed that the bacteria from the eight patients were of the same strain as those from the surface of the dental treatment room. After treating the patients under a revised infection control protocol including single use barrier covers, MRSA was not detected on the surfaces of the dental treatment room, and no nosocomial infection or colonization occurred. These results suggest that MRSA contaminates the surfaces of the dental treatment room, and therefore should be considered a possible reservoir of MRSA.
Hospital and nursing home dentists routinely provide care for patients who are at high risk for acquiring MRSA colonization and/or infection. These HCWs may be a source of transmission of MRSA to patients, to family members, and to the community. One study5 shows a significant transfer of staph occurs from the patient's mouth to the operator's fingers, and from the operator's fingers to environmental surfaces. Two healthy dental patients developed MRSA infections after being treated by a dentist who was a carrier and had acquired the organism in a hospital one year before. The dentist had failed to wear gloves when treating these two patients.
Because there is a risk of cross-infection of MRSA between patients, dental healthcare workers (DHCW) should follow the infection control recommendations issued by the CDC. These standard precautions include the use of barriers, personal protective attire, and appropriate hand hygiene protocols.
Hand Hygiene Products
There are a variety of preparations used for hand hygiene from plain soap to waterless antiseptic agents (Table 1). In the United States, antiseptic hand washing products intended for use by HCWs are regulated by the Food and Drug Administration's (FDA) division of Over-the-Counter Drug Products.
FDA is undertaking a review of active ingredients used in a variety of (OTC) antiseptic rubs and wash products. Health care antiseptics are being evaluated separately from consumer antiseptics because they have different proposed use settings and target populations, and the risks for infection in the different settings varies.
• Patient preoperative skin preparation - fast-acting, broad spectrum, and persistent antiseptic-containing preparation that substantially reduces the number of microorganisms on intact skin.
• Antiseptic hand wash or HCW hand wash - antiseptic containing preparation designed for frequent use; it reduces the number of microorganisms on intact skin to an initial baseline level after adequate washing, rinsing, and drying; it is broad-spectrum, fast-acting, and if possible, persistent*.
• Surgical hand scrub - antiseptic-containing preparation that substantially reduces
the number of microorganisms on intact skin; it is broad-spectrum, fast-acting, and persistent*.
*Persistent Activity - may be demonstrated by sampling a site several minutes or hours after application and demonstrating bacterial antimicrobial effectiveness when compared with a baseline level. This property also has been referred to as "residual activity." Both substantive and non-substantive active ingredients can show a persistent effect if they substantially lower the number of bacteria during the wash period.
Plain soaps are detergent-based products that can remove dirt, soil and other organic materials from the hands. Plain soap and water can remove transient flora from the hands but have minimal antimicrobial activity and little effect on the resident flora.8
The preferred products for hand hygiene depend on the type of procedure, the degree of contamination, and the desired persistence of antimicrobial action on the skin. For routine dental examinations and nonsurgical procedures, hand washing and hand antisepsis is achieved by using plain or antimicrobial soap and water.
If the hands are not visibly soiled, an alcohol-based hand rub may be used. When no sinks are available, a generous amount can be applied to the hands and then rubbed together until the gel or foam dries. Studies have shown that alcohol products can reduce viable bacteria counts and aid in the frequency of hand hygiene due to ease of use. It is important to note that periodic (after 4 - 5 hand rub procedures) hand washing is necessary to remove debris build up.8
The purpose of surgical hand antisepsis is to eliminate transient flora and reduce resident flora for the duration of a procedure to prevent introduction of organisms in the operative wound if gloves become punctured or torn. Skin bacteria can rapidly multiply under surgical gloves if hands are washed with soap that is not antimicrobial. Agents used for surgical hand antisepsis should substantially reduce microorganisms on intact skin, contain a nonirritating antimicrobial preparation, have a broad spectrum of activity, be fast-acting, and have a persistent effect.6
Antiseptic Hand Hygiene Products
An antiseptic hand wash is performed with water and an antimicrobial soap. Examples of antimicrobial soaps include: alcohols, chlorhexidine, chlorine, hexachlorophene, iodine, chloroxylenol [also known as para-chlorometaxylenol (PCMX)], quaternary ammonium compounds, and triclosan. These liquid soaps are stored in closed containers. The container should be disposable or completely washed and dried before refilling. Containers should never be "topped off" as this can allow transfer of contaminates to the new soap. The purpose of an antiseptic hand wash is to remove soil and transient microorganisms and reduce resident flora.
Antiseptic hand rubs are waterless, alcohol based products that include antiseptics such as chlorhexidine, quaternary ammonium compounds, octenidine, or triclosan. The purpose of an antiseptic hand rub is to remove or destroy transient microorganisms and to reduce resident flora. The antimicrobial activity of alcohols can be attributed to their ability to denature proteins. Alcohol solutions containing 60%-95% alcohol are most effective. Higher concentrations are less potent because proteins are not denatured easily in the absence of water.1 Alcohols are rapidly germicidal when applied to the skin but are not appropriate for use when hands are visibly soiled or contaminated with proteinaceous materials.
Surgical Hand Hygiene Products
Surgical antisepsis is performed with water and antimicrobial soap containing products such as chlorhexidine, iodine and iodophors, chloroxylenol or triclosan or water and plain soap followed by an alcohol-based surgical hand-scrub with persistent activity. Immediate and persistent activity is considered the most important factor in determining the efficacy of the product. U.S. guidelines recommend that agents used for surgical hand scrubs should substantially reduce microorganisms on intact skin, contain a nonirritating antimicrobial preparation, have broad-spectrum activity, and be fast-acting and have a persistent effect.1 Persistent activity is critical because microorganisms can colonize on hands in the moist environment underneath gloves.6
Antiseptic preparations intended for use as surgical hand scrubs are evaluated for their ability to reduce the number of bacteria released from hands at different times, including:
• immediately after scrubbing
• after wearing surgical gloves for 6 hours (i.e., persistent activity)
• after multiple applications over 5 days (i.e., cumulative activity)
Hand Washing Techniques
Aseptic techniques involve mechanical and manual protocols. Whenever possible, mechanisms should be installed so the DHCW does not need to touch handles. Foot pedals, elbow levers, or "electronic eyes" should be installed to aid in the process of hand washing. Soap in squeeze bottles or soap bars should not be used in the dental setting.8
DHWC should perform hand washing at the following times throughout the work day:
• beginning of day
• when hands are visibly soiled
• after bare-touching contaminated surface/object
• before donning gloves
• after removing gloves
• before touching eye contacts
• before leaving the restroom
• before eating/handling food
• end of day8
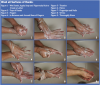
Effective hand washing (Table 2, Figure 1 through Figure 9) is accomplished by wetting hands and applying soap or an antimicrobial/antiseptic agent. The next step includes vigorously rubbing hands together to create a lather covering all the surfaces of the palms, tops of the hands, between the fingers, base of the fingers and thumbs, backs of the fingers, wrists and fingernails. This step should take at least 15 seconds. Then rinse hands thoroughly to remove all the lather. Washing and rinsing should be performed with cool water as hot water can be drying to the skin. If handle-less faucets are not available, a barrier must be used so as not to re-contaminate the hands when turning off the water.
Hands should be thoroughly dried with disposable toweling. The DHCW should first dry the hands, then forearms. This would be the appropriate time to turn off a running hand-controlled faucet with a barrier. The toweling should be dropped into the waste receptacle and not pushed into the receptacle with the newly washed hands.
To facilitate better hand washing and ease of donning gloves, fingernails should be no longer than one-quarter inch from the nail bed. Presence of gram-negative organisms is greater among wearers of artificial nails than among non wearers, both before and after hand washing. In addition, artificial fingernails or extenders have been epidemiologically implicated in multiple outbreaks involving fungal and bacterial infections in hospital intensive-care units and operating rooms. Freshly applied nail polish on natural nails does not increase the microbial load from periungual skin if fingernails are short. However, chipped nail polish can harbor added bacteria.6
Wearing jewelry such as rings is not recommended by the CDC and makes effective hand hygiene more difficult. Several studies have demonstrated that skin underneath rings is more heavily colonized than comparable areas of skin on fingers without rings. One study found that 40% of nurses harbored gram-negative bacilli on skin under rings and that certain nurses carried the same organism under their rings for several months.1 Rings can also cause tears in gloves thus contaminating the hands.
The amount of time spent washing hands is a critical aspect in reducing the transmission of pathogens to other people and environmental surfaces (Table 3). Drying hands thoroughly is also important. Wet hands have been known to transfer pathogens much more readily than dry hands or hands not washed at all. The residual moisture determines the level of bacterial and viral transfer following hand washing.7
The primary function of the skin is to reduce water loss to the body, to provide protection against abrasive action and microorganisms, and to act as a permeability barrier to the environment.1 Intact skin also helps to prevent disease transmission to the DHCW (Table 4, Figure 10 through Figure 12). Lotions with a base of aloe vera, glycerin, or vitamins A or E are often recommended to ease the dryness resulting from frequent hand washing. However, petroleum-based lotion formulations can weaken latex gloves and increase permeability. For that reason, lotions that contain petroleum, lanolin, or other oil emollients should only be used at the end of the work day.6
Selection of Gloves
The Occupational Safety and Health Administration (OSHA) mandates that the DHCW wears gloves during all patient-care activities that may involve exposure to blood or OPIM (other potentially infectious materials). In addition to protecting the DHCW, gloves also reduce the chance of transmission of microorganisms from the DHCW to the patient. Gloves do not replace the need for proper hand hygiene, but are used in conjunction with hand hygiene.
Gloves used by DHCWs are usually made of natural rubber latex and synthetic non latex materials (e.g., vinyl, nitrile, and neoprene). In addition to different types of glove materials and sizes, there are two different types of glove fit. Ambidextrous gloves fit either hand; and "right/ hand" "left/hand" gloves are hand specific. Wearing hand specific gloves may be more comfortable as they allow the DHCW's hands to rest in a more relaxed position than ambidextrous gloves.
Because gloves are task-specific, their selection should be based on the type of procedure to be performed (e.g., surgery or patient examination). Sterile surgeon's gloves must meet standards for sterility assurance established by the FDA and are less likely than patient examination gloves to harbor pathogens that could contaminate an operative wound.6 Heavy-duty utility gloves (Figure 13) are both chemical and puncture resistant and are a better choice when decontaminating instruments or environmental surfaces. Polyethylene over gloves (food handler gloves) (Figure 14) can be worn over patient examination gloves to eliminate multiple glove changes when it is necessary to retrieve needed items while treating a single patient.
Gloves are manufactured as single-use disposable items that should be used for only one patient, and then discarded. In addition to donning new gloves for each patient, gloves must be replaced when torn or punctured. Washing gloves is not recommended because it can lead to the formation of glove micro punctures. A condition called wicking can allow penetration of liquids through these undetected holes and can cause hand contamination.
Wearing gloves does not eliminate the need for hand washing as gloves do not provide complete protection against hand contamination. Pathogens may gain access to the DHCW hands via small defects in gloves or by contamination of the hands during glove removal. Limited studies of the penetrability of different glove materials under conditions of use have been conducted in the dental environment. Consistent with observations in clinical medicine, leakage rates vary by glove material (e.g., latex, vinyl, and nitrile), duration of use, type of procedure performed, as well as by manufacturer.6
Hands should always be washed both before and after glove use. While the skin is tightly covered, resident flora dramatically increases, possibly as great as 4000-fold per hour. Hand washing before and after glove use reduces the amount intially. Washing afterward removes the developed resident flora plus removes residual latex proteins, and sweat which leads to possible skin irritations.8
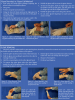
Surgical Glove Donning Techniques
Some dental procedures require the use of sterile gloves. When using sterile gloves, specific techniques for placement are required to ensure that the sterility of the gloves is not compromised. There are two techniques for donning sterile gloves and each should be practiced as there are specific steps to follow. The first technique is called "Closed Donning" or "Closed Cuff Method" (Table 5, Figure 15 through Figure 20). The second technique is called "Open Donning" or "Open Cuff Method" (Table 6, Figure 21 through Figure 28).
Summary
DHCWs have an obligation to prevent the spread of HAIs. Adhering to proper hand hygiene procedures, selecting appropriate hand hygiene products, and the use of gloves are essential components of infection control. DHCW must also protect themselves by recognizing pitfalls such as irritants or allergies that may pose obstacles to proper hand hygiene. Occupational irritants and allergies can be caused by frequent hand washing, exposure to hand hygiene products, exposure to chemicals, and shear forces associated with wearing or removing gloves.
Since the primary defense against infection and transmission of pathogens is healthy, unbroken skin, DHCWs must take steps to ensure that their skin remains healthy and intact. These steps include evaluating different types of hand hygiene products, gloves and lotions for the best compatibility. Products that have low irritancy potential are more likely to be utilized by DHCWs. If the DHCW sees a breakdown of his/her skin barrier, steps should be taken to determine the cause and remedy. If contact dermatitis or latex sensitivity is suspected, a diagnosis by a qualified medical practitioner should be sought.
Glossary
alcohol-based hand rubs - alcohol-containing preparation designed for application to the hands for reducing the number of viable microorganisms on the hands; in the United States this usually contains 60%-75% ethanol or isopropanol
antimicrobial soap - soap (i.e., detergent) containing an antiseptic agent
antiseptic agent - antimicrobial substances that are applied to the skin to reduce the number of microbial flora
antiseptic hand wash - washing hands with water and an antimicrobial soap or other detergent containing an antiseptic agent
antiseptic hand rub - applying an antiseptic hand rub product to all surfaces of the hands to reduce the number of microorganisms present
colonized - bacteria are present but not causing infection
detergent - compounds that possess a cleaning action; composed of both hydrophilic and lipophilic parts and can be divided into four groups: anionic, cationic, amphoteric, and nonionic
efficacy - power to produce an effect
EPA - Environmental Protection Agency
FDA - Food and Drug Administration
hand antisepsis - either antiseptic hand wash or antiseptic hand rub
hand hygiene - general term that applies to hand washing, antiseptic hand wash, antiseptic hand rub, or surgical hand antisepsis
hand washing - washing hands with plain (i.e., non-antimicrobial) soap and water
latex - milky fluid extracted from rubber trees; used to make flexible plastics and gloves
nosocomial infections - result of treatment in a hospital or a health care facility
puerperal fever - "childbed fever" a serious form of septicemia contracted by a woman during childbirth or abortion (usually attributable to unsanitary conditions); formerly widespread but now uncommon
persistent activity - prolonged or extended antimicrobial activity that prevents or inhibits the proliferation or survival of microorganisms after application of the product
periungual - area surrounding a fingernail
plain soap - detergents that do not contain antimicrobial agents or contain low concentrations of antimicrobial agents that are effective solely as preservatives
substantivity - certain active ingredients that adhere to the stratum corneum (i.e., remain on the skin after rinsing or drying) to provide an inhibitory effect on the growth of bacteria remaining on the skin
surgical hand antisepsis - antiseptic hand wash or antiseptic hand rub performed preoperatively by surgical personnel to eliminate transient flora and reduce resident hand flora; antiseptic detergent preparations often have persistent antimicrobial activity
visibly soiled hands - showing visible dirt or visibly contaminated with proteinaceous material, blood, or other body fluids (e.g., fecal material or urine)
waterless antiseptic agent - antiseptic agent that does not require use of exogenous water; after applying such an agent, the hands are rubbed together until the agent has dried
*The majority of these definitions are from the 2002 CDC Guideline for Hand Hygiene in Health-Care Settings, pgs 3-4. Revisited January 17, 2014.1
Additional Reliable Resources
Centers for Disease Control and Prevention (www.CDC.gov)
• Guideline for Hand Hygiene in Health-Care Settings - 2002
• Guidelines for Infection Control in Dental Health-Care Settings - 2003
The Organization for Safety, Asepsis and Prevention (OSAP.org)
The Associated for Professionals in Infection Control and Epidemiology (APIC.org)
The following website shows step-by-step illustrations for using and removing sterile gloves: http://www.brooksidepress.org/Products/Scrub_Gown_and_Glove_Procedures/lesson_1_Section_6.htm
References
1. Boyce, John. "Guideline for Hand Hygiene in Health- Care Settings." Morbidity and Mortality Weekly Re- port 51(RR16)25 Oct 2002 27.1-30 Jan 2014
2. MRSA in Healthcare Settings. CDC Infection Control in Healthcare. 03OCT2007. Centers for Disease Control. 27 Sep 2008
3. Rautemaa, R, Nordberg A, Wuolijoki-Saaristo K and Meurman JH. Bacterial aerosols in dental practice-a potential hospital infection problem? J Hosp Infect 64: 76-81,2006.
4. Kurita H, Kurashina K, Honda T. Nosocomial transmission of methicillin-resistant Staphylococcus aureus via the surfaces of the dental operatory. Br Dent J. 2006 Sept 9;201(5):297-300; discussion 291.
5. Antonelli JR, Valenza JA. Methicillin-resistant Staphylococcus aureus: a crisis in infection control. Compendium. 1993 Jan;14(1):22,24,26-30.
6. Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care setting-2003. MMWR Recomm Rep. 2003;52(RR-17):15-18 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm.
7. "OPRP - General information on Hand Hygiene." Centers for Disease Control and Prevention 11 Oct 2008
8. Miller, CH. (2014). Infection Control and Management of Hazardous Materials for the Dental Team. St. Louis. Elsevier. 5th ed. pgs. 99-106.
9. Antibiotic Resistance Threats in the United States. 2013. Centers for Disease Control. 1.22.14 http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. pg. 77.
10. National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination. http://www.health.gov/hai/prevent_hai.asp#hai. Accessed February 27, 2014.
About the Author
Leslie Canham, CDA, RDA
Leslie Canham, CDA, RDA, has been in the dental field since 1971. She is a Certified and Registered Dental Assistant and a nationally recognized speaker and consultant on Infection Control and OSHA compliance. She is authorized by the Department of Labor as an OSHA Outreach Trainer. Leslie is a member of several professional organizations including: Organization for Safety, Asepsis and Prevention (OSAP), Speaking Consulting Network (SCN), Academy of Dental Management Consultants (ADMC), National Speakers Association (NSA), California Dental Association (CDA), American Dental Assistants Association (ADAA), and the California Dental Assistants Association (CDAA).
Leslie has authored several articles and continuing education courses that have appeared in numerous dental publications. Leslie is founder of Leslie Canham & Associates providing consulting, on-site training, mock inspections, home-study courses, teleconferences and webinars.
Special thanks to Mike Canham of Leslie Canham & Associates for the hand washing and glove illustrations.