You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The dental profession has concentrated over the last several decades on reducing caries incidence and periodontal diseases; as a result, these dental conditions have decreased to a level where more patients are seeking esthetic improvements instead.1 One of the most popular requests today in esthetic dentistry is for treatment of discolored and hypoplastic dentition. Patients' demand for an improved smile appearance, especially a whiter smile, has made tooth whitening a very common dental procedure in most practices today. Tooth whitening, commonly referred to as "tooth bleaching," provides a more conservative treatment option than resin-bonded composites, veneers, or crowns for patients who have discolored teeth.2,3 Ideal candidates for tooth-whitening procedures include patients whose teeth are stained due to the aging process, chromogenic stain-causing food and beverages, endodontic treatment, tetracycline taken during tooth development, and tobacco product use. The success of today's tooth-whitening treatment depends on the type and intensity of the discoloration, the location on the teeth, and a careful examination and diagnosis by the practitioner.
History
There have been many attempts throughout history to find an effective tooth-whitening method. Whiter teeth were desired at least as far back as 2000 years ago. Roman physicians claimed during the 1st century that brushing the teeth with urine would whiten the teeth.4 As recently as the late 18th century, a solution of nitric acid was used by barber-surgeons to whiten the teeth after abrading the enamel with a coarse metal file. In 1895, the combination of pyrozone 25% and electricity was reported to whiten endodontically treated teeth.5 In 1916, endemic fluorosis was treated with hypochloric acid.6 In 1939, the use of 30% hydrogen peroxide, ether, and heat was suggested to treat fluorosis staining.7 In 1966, the combination of hydrochloric acid and hydrogen peroxide was recommended to remove "brown stain from mottled teeth" due to chronic endemic dental fluorosis.8 These early efforts to whiten teeth shared the common assumption that the tooth-whitening process required the removal of extrinsic enamel stain, although the actual mechanism of action was not well understood.
In 1970 Cohen and Parkins first published a method for whitening the teeth of young adults with cystic fibrosis.9 These patients had undergone tetracycline treatment resulting in discolored dentin. This published method suggested that tooth whitening using hydrogen peroxide involves penetration to the dentin. In 1976, the "walking bleach" technique was introduced by Nutting and Poe. This technique for whitening non-vital teeth required the use of 35% hydrogen peroxide and sodium perborate.10 The modern technique for vital-tooth whitening via a nightguard (or tray) was introduced in 1989, published by Haywood and Heymann.11 This procedure is widely used in dental practices today as the take-home system of tooth whitening.
Mechanism of Action
Many studies have been completed regarding the effectiveness of different tooth-whitening materials and techniques, specifically carbamide peroxide and hydrogen peroxide.12-26 Although the exact mechanism by which tooth whitening occurs is not completely understood, it is known that carbamide peroxide decomposes into hydrogen peroxide, and hydrogen peroxide is able to penetrate through the organic matrix of enamel and dentin.27-29 Hydrogen peroxide is an oxidizing agent, producing very reactive free radicals.30 The radicals have unpaired electrons, making them unstable and causing them to break down carbon double bonds and form single bonds. These simpler molecules reflect light, creating a successful whitening action.
Bleaching Penetration
Carbamide peroxide breaks down into hydrogen peroxide, carbon dioxide, urea, and ammonia. Peroxide is able to penetrate the dentinal tubules, and very small amounts may reach the pulp and lead to a reversible pulpitis.27,28,31 It is important to note that 10% carbamide peroxide is equivalent to about 3.5% hydrogen peroxide; whitening products are available in both formulations.32 Studies have shown that carbamide peroxide gel is able to alter dentin color over time.33
Tooth Discoloration: External-Internal Staining
Discoloration of the teeth can be caused by superficial changes in the enamel surface or deep internal staining affecting more of the tooth structure. Identifying the type of discoloration and diagnosing the cause can aid the dentist in developing an appropriate treatment plan for the patient. Vital-tooth discoloration can result from aging, tobacco use, chromogenic food and beverages, and medications.34
The staining effect of tea, coffee, red wine, and tobacco products generally results in yellow, brown, or black staining. Internal staining typically results from improper calcification and/or hypoplasia. Causes of more severe discolorations include medication taken during tooth formation, excessive fluoride intake during enamel formation, and calcification, systemic conditions, dental conditions, and the aging process. In 1956, Schuster and Shwachman reported on tooth discoloration caused by the incorporation of systemic tetracycline into tooth structure.35 Tetracycline discoloration is caused by the systemic intake of tetracycline during tooth formation in utero through age 8. The extent of discoloration is dependent on the amount and duration of tetracycline intake and is incorporated into the dentin during calcification.36,37 Gray, blue, brown, and yellow colors may be seen in a banding formation. Tetracycline staining responds to tooth whitening, but daily treatment must often be extended to 3 to 6 months with a take-home whitening system.
The intake of excessive systemic fluoride during enamel formation and calcification can lead to a defective matrix and calcification, often referred to as mottled enamel.38,39 Fluorosis typically appears as bilateral chalky white spots or yellow or brown staining.
Certain systemic conditions can also cause discoloration of the teeth. Amelogenesis imperfecta causes hypoplasia or hypocalcification with yellow or brown stains, and dentinogenesis imperfecta results in brownish violet, yellowish, or gray discolorations.40 Hypoplasia or hypocalcification can also occur with other systemic conditions.40-43
Previous dental treatment may lead to darkening of the teeth as materials age or due to their intrinsic color (eg, pins, posts). Caries can also lead to discolorations of the teeth. The aging process generally involves thinning of the enamel, thinning or loss of the outer translucent enamel layer, and secondary dentin formation, creating a darker appearance.41
Tooth-Whitening Protocol
Professionally supervised tooth whitening can be safe and effective. An oral examination provides the practitioner an opportunity to determine the severity and type of discoloration and determine the appropriate treatment plan. Medication-related staining will need longer and more persistent treatment than stains related to aging, tobacco, or eating habits. Determination of caries, restorations, or endodontic treatment is important before tooth whitening.23 Exposed dentin, faulty restorations, and microcracks in enamel should be identified and treated before whitening because unsealed restorations and caries can result in extensive sensitivity.41
Gingival irritation and tooth sensitivity are the two main complaints during tooth-whitening procedures. Generally, tooth whitening is well tolerated, but some patients may experience tooth sensitivity or mild soft-tissue irritation. Both in-office and take-home bleaching procedures have been reported to induce sensitivity in a significant number of patients.44 These symptoms are typically mild and resolve after the end of treatment.
The fabrication of a custom-fitted, well-adapted whitening tray for take-home whitening is important. Tray fabrication may include "scalloping" of the gingival margin to decrease the possibility of bleaching agent going onto the soft tissue. Tray material thickness is important to prevent jaw pain, occlusal interference, and other problems associated with the temporomandibular joint.45 Tray design and whether to include reservoirs should be determined based on the viscosity of the tooth-whitening material and manufacturer recommendation; however, clinical studies have shown no difference in tooth whitening using 10% carbamide peroxide with or without reservoirs.46 A relatively neutral-pH tooth-whitening material is important for take-home whitening systems because enamel demineralization, erosion, and root resorption may occur at pH below 5.2.47
Immediate placement of composite resin on whitened teeth is discouraged. One reason is to ensure shade stability because the teeth may be dehydrated after whitening, but there are also studies that suggest peroxide-based whitening systems can negatively affect bonding procedures for 4 days.48,49
Bleaching Side Effects
Toxicology
In vitro evaluation of whitening agents including 10% carbamide peroxide and 4% hydrogen peroxide showed their toxicologic effect to be comparable to or less than common dental materials such as eugenol, dentifrices, mouthrinses, and composites.50 Daily exposure of carbamide peroxide should not exceed 10 mg/kg.51 Li reported 502 mg per application is the average amount of bleaching agent used, equivalent to 8.37 mg/kg.50 Because patients would be likely to swallow only a small amount of the applied whitening agent, the material is very safe.
Oral Side Effects
Gingival irritation may occur due to poorly fitted trays, excessive amount of whitening agent applied to home trays, or wearing trays longer than the recommended time.52 Irritation is generally minimal and resolved either by adjustment of the tray or waiting until treatment completion.53
Tooth Sensitivity
Studies show that sensitivity may occur in as many as 55% to 75% of patients during whitening treatment. Interestingly, placebo groups also experienced 20% to 30% sensitivity. In one study, 15% of participants reported sensitivity wearing only the bleaching tray without any whitening agent.54,55 Tooth sensitivity during whitening can be multifactorial. Tray fabrication, overzealous toothbrushing, and tooth dehydration are likely factors; free-radical formation, allergies, and chemical sensitivities may also be involved.55-57 Both potassium-nitrate desensitizers and sodium fluoride were shown to be effective for the treatment of whitening sensitivity in a recent systematic review and meta-analysis.58
Tooth-Whitening Systems
Tooth whitening using varied concentrations of peroxide (both hydrogen peroxide and carbamide peroxide) has been demonstrated to be safe and effective59 when manufacturers' instructions are followed. Peroxide-based tooth whitening can be performed in a variety of regimens, including in-office procedures, dentist-prescribed and supervised home treatments, and over-the-counter systems.50 Dental practices in the United States commonly offer some form of tooth-whitening system, with many offering several options.
1. Whitening Take-Home System
The length of treatment for take-home whitening varies, based on the tooth color at the start of the whitening process. Most common is 2 to 4 weeks.11,45 Hydrogen peroxide for the removal of intrinsic stains from vital teeth and whitening teeth has been used for years, with the first article published on nightguard vital bleaching by Haywood and Heymann in 1989.11 A model is made from the patient's teeth, and a fitted tray is fabricated from a soft-plastic nightguard material. A small amount of carbamide peroxide gel is loaded into the tray and seated over the patient's teeth for 1 hour to overnight. The carbamide-peroxide gel concentration varies from 10% to 22%.
Compared with in-office whitening options, the take-home system has the advantage of decreased cost. Less chair time is required because the patient self-administers the dentist-prescribed whitening gel at home. Disadvantages with take-home tooth-whitening systems include the following: (1) patient compliance with the daily application of carbamide-peroxide gel; (2) tray wear-time compliance for the number of applications and hours or overnight as recommended; and (3) 4 or more weeks may be required for the treatment.
2. In-Office System Without a Light
In-office tooth whitening techniques generally use a hydrogen-peroxide bleaching agent ranging from 15% to 35% concentration. Patients must be made aware that this procedure typically requires a take-home component for touch-ups. There are advantages to in-office whitening procedures: patient compliance need is minimal (and supervised), and immediate results are visible. However, the dental chair-time requirement and consequent increased cost to the patient are disadvantages.
3. Light-Activated Whitening Systems for In-Office Use
Public demand for light-activated (enhanced) tooth-whitening systems has been fueled by the introduction of light-activated devices by dental manufacturers and their marketing. Plasma arc, light-emitting diodes (LED), argon lasers, and other devices have increased patient awareness of quick tooth-whitening systems. A recent laboratory study concluded that LED lamps are the preferred method of light activation for tooth whitening over halogen lamps and lasers because LED lamps produced a significant color change but only slightly increased pulpal temperature.60
Hydrogen peroxide has been used for whitening teeth with or without heat or light activation. The technique for using hydrogen peroxide and a hand-held heating source for bleaching discolored teeth was introduced by Cohen and Parkins.9 Use of light or heat may accelerate the degradation of hydrogen peroxide and reduce necessary treatment time. While the teeth are lightened more rapidly, degradation of the hydrogen-peroxide tooth-whitening material must occur within clinically tolerable heat levels.
4. Combination Technique
The combination of in-office and take-home tooth-whitening systems reduces chair time and may reduce the number of office visits, thereby reducing patient cost versus in-office tooth-whitening alone.61 Therefore, the combination method may increase success and patient satisfaction with the tooth-whitening results. The in-office tooth-whitening treatment uses high-concentration hydrogen peroxide chairside, which is followed by a take-home treatment and may include an additional in-office tooth-whitening treatment.24
Over-the-Counter Products
Many over-the-counter tooth whitening products are available. Examples include whitening toothpastes, whitening strips, tray-based, and brush-on whiteners. Some systems now include lights (usually LEDs) intended to activate the whitening agents, although little research on these systems is available in scientific literature. One clinical study in particular showed that maintenance of whitening was improved by two Vita shades at 6 months after whitening through the use of a powered toothbrush rather than a manual toothbrush.62
Toothpaste
Many whitening toothpastes are on the market. Of these, few show effectiveness in stain removal. Often these toothpastes contain no bleaching agents or very low bleaching-agent concentration (1% or less peroxide).63 In most cases, the contact time with the teeth may be too short to be effective at overall tooth whitening.12 Many whitening toothpastes contain abrasives for the removal of surface stains, resulting in a whiter appearance, and may in combination with low percentages of carbamide peroxide or hydrogen peroxide lead to one or two shade changes.59 Some whitening toothpastes now contain optical agents such as blue covarine. These optical agents can help teeth appear whiter after brushing.64 Recently, charcoal toothpastes have been marketed for their tooth-whitening ability; however, a recent literature review reported insufficient data to confirm either safety or efficacy.65
Whitening Strips
Over-the-counter tooth-whitening strips are widely available. These strips come in various strengths and may be recommended for 30 minutes to 1 hour once or twice daily. The flexible strips (often peroxide-impregnated polyethylene) allow whitening over short treatment times. This method may be helpful for maintenance of previously whitened teeth. A clinical study showed that teeth treated with a 5.3% hydrogen-peroxide polyethylene whitening strip demonstrated nearly three shade changes versus placebo.66 Patients who cannot afford in-office tooth-whitening treatment or who cannot make multiple dental visits for tray fabrication may choose this option.45 According to a 2000 study, even tetracycline-stained teeth may be whitened with tooth-whitening strips.45
Tray-based
Over-the-counter, tray-based tooth-whitener kits usually include prefabricated whitening trays, which are not ideal because the trays do not fit as well as in-office custom trays. The poor fit can lead to leakage of the bleaching material onto soft tissues, causing irritation and generalized discomfort. The bleaching-agent formulation may be less desirable than formulas dispensed by dentists.67
Brush-on Whiteners
Brush-on tooth whiteners offer a paint-on application system. This nontray-based treatment can be applied to the teeth while avoiding the gingiva, reducing soft-tissue irritation. Bleaching-agent concentration varies, but it is generally lower than dentist-dispensed materials. Little research is available on these systems.
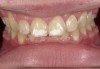
Case Study 1
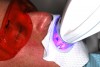
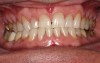
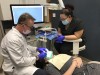
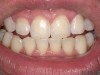
A 55-year-old male patient with no medical contraindications requested tooth whitening (Figure 1 and Figure 2). The patient had no interest in extended dental care, had a healthy dentition, and simply wanted his teeth whitened. Medical and dental histories were reviewed. There were no further dental treatment needs or contraindications to whitening. The treatment plan was presented to the patient and options were discussed. Treatment consisted of 1-hour light-activated chairside whitening (Figure 3); 25% hydrogen peroxide gel was applied 4 times at 15-minute intervals. The patient was pleased with the whitening achieved in such a short time period; the immediate postoperative photograph showed a change of approximately 7 shades (Figure 4). After the procedure, postoperative instructions were given to the patient, along with a take-home whitening system to maintain his whitening results (Figure 5).
Case Study 2
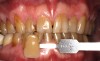
A 16-year-old healthy male was seen immediately after 4 years of active orthodontic treatment. Both the patient and his mother expressed concern regarding the appearance of the teeth. The patient's teeth had white-spot lesions, hypocalcifications, and an overall dark color (Figure 6). After a complete dental examination, the treatment plan included both microabrasion and light-activated in-office whitening.
Microabrasion is a dental technique introduced by Theodore P. Croll, DDS, of Doylestown, Pennsylvania, in the 1980s. The technique uses hydrochloric acid with an abrasive to remove surface-enamel discolorations.68 One clinical study showed that microabrasion is most successful when treating single-line or patched enamel defects but not as successful when attempting to treat multiline or diffused types of enamel defects.69 In a 1990 article, Croll stated that although microabrasion is contraindicated for dentinal discolorations including tetracycline staining and dentinogenesis imperfecta, it should be attempted when the depth of the enamel defect is unknown.70 If necessary, composite bonding can be performed, but the microabrasion alone can correct many surface defects.70 Interestingly, microabrasion is referred to as "simultaneous abrasion and erosion" and results in insignificant microscopic enamel loss.71 Studies have shown that microabrasion is a conservative treatment, with 25 to 200 m enamel loss noted after up to 12 applications for 30 seconds.72
The standard microabrasion technique is to isolate with a rubber dam and seal the margins with petroleum jelly and then apply the hydrochloric-acid material to the stained area or surface irregularities with a rotary mandrel and rubber tip or cup, or cotton swab, for intervals up to 30 seconds, rinsing with water.72 After the treatment, the teeth should be thoroughly rinsed and polished, and a sodium-fluoride gel should be applied for 4 minutes to aid in remineralization.72
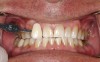
In the case study, photographs were taken and a rubber dam was placed, isolating the teeth to be treated. Microabrasion was completed to attempt to eliminate the white-spot lesions using a 10% hydrochloric-acid/silicon-carbide gel microabrasion kit (Figure 7 and Figure 8). The teeth were microabraded manually using cotton swabs and hydrochloric-acid gel for 10-second intervals and then immediately rinsed with water. After five to seven applications of gel per tooth (with water irrigation), a significant decrease in chalkiness was noted, with no sensitivity or gingival irritation. The patient was then given a break to relax and allow the teeth to rehydrate (Figure 9). Next the patient was treated with three 15-minute sessions of 25% hydrogen-peroxide gel light-activated in-office whitening. The patient experienced mild tooth sensitivity after whitening, so a potassium-nitrate/sodium-fluoride gel for sensitivity was brushed onto the teeth. After about 3 minutes, the patient spit the gel out, reporting the sensitivity was greatly diminished and disappeared completely in a few minutes. The patient and mother were extremely pleased with the final result (Figure 10). The patient was provided with a take-home whitening system for follow-up and maintenance (Figure 11).
Summary
Tooth whitening is a popular dental procedure but is not well understood by dental professionals or patients. Understanding the basic mechanism of action, safety considerations, and systems available can help dentists appropriately treatment plan their patients for whitening procedures. Although it may not be possible to determine the exact cause of sensitivity during whitening treatment, performing a thorough dental examination can help avoid common causes, and additional sensitivity treatments are effective.
Extensive research has been published on the safety and efficacy of standard whitening systems. As new systems come to market, it is especially important to review their research. It is recommended that well-researched materials are chosen to ensure their safety and efficacy. Tooth whitening can be included in combination with other dental treatments for optimal results. Dental microabrasion combined with tooth whitening can be effective in treating white-spot lesions.
ABOUT THE AUTHORS
Connie Kugel, RDH, BS
Boston Center for Dental Education
Boston, Massachusetts
Gerard Kugel, DMD, PhD, MS
Trinity Dental Practice & Boston Center for Dental Education
Boston, Massachusetts
REFERENCES
1. Burrell KH. ADA supports vital tooth bleaching-but look for the seal. J Am Dent Assoc. 1997;128(suppl):3S-5S.
2. Papathanasiou A, Bardwell D, Kugel G. Combining in office and take home whitening systems. Contemp Esthet Restor Pract. 2000;4(8);88-91.
3. Papathanasiou A, Bardwell D, Kugel G: A clinical study evaluating a new chairside and take-home whitening system. Compend Contin Educ Dent. 2001;22(4):289-298.
4. Dale BG, Aschheim KW. Esthetic Dentistry: A Clinical Approach to Techniques and Materials. Philadelphia, PA: Lea & Febiger; 1993:205-206.
5. Westlake A. Bleaching teeth by electricity. Am J Dent Sci. 1895;29:101.
6. Adams TC. Enamel color modifications by controlled hydrochloric acid pumice abrasion: a review with case summaries. J Indiana Dent Assoc. 1987;66(5):23-26.
7. Younger HB. Bleaching fluorine stain from mottled enamel. Texas Dent J. 1939;57:380.
8. McInnes J. Removing brown stain from teeth. Ariz Dent J. 1966;12(4):13-15.
9. Cohen S, Parkins FM. Bleaching tetracycline-stained vital teeth. Oral Surg Oral Med Oral Path. 1970;29(3):465-471.
10. Nutting EB, Poe GS. A new combination for bleaching teeth. Dent Clin North Am. 1976;10:655-662.
11. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-176.
12. Sharif N, MacDonald E, Hughes J, et al. The chemical stain removal properties of ‘whitening' toothpaste products: studies in vitro. Br Dent J. 2000;188(11):620-624.
13. Mokhlis GR, Matis BA, Cochran MA, Eckert GJ. A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime use. J Am Dent Assoc. 2000;131(9):1269-1277.
14. Christensen GJ. Tooth bleaching, home-use products. Clin Res Assoc Newsletter. 1989;13(7):1-3.
15. Cibirka RM, Myers M, Downey MC, et al. Clinical study of tooth shade lightening from dentist-supervised, patient-applied treatment with two 10% carbamide peroxide gels. J Esthet Dent. 1999;11(6):325-331.
16. Jones AH, Diaz-Arnold AM, Vargas MA, Cobb DS. Colorimetric assessment of laser and home bleaching techniques. J Esthet Dent. 1999;11(2):87-94.
17. Haywood VB: Nightguard vital bleaching: history and products update, part 1. Esthet Dent Update. 1991;2(4):63-66.
18. Haywood VB: Nightguard vital bleaching: history and products update, part 2. Esthet Dent Update. 1991;2(5):82-85.
19. Haywood VB, Heymann HO: Nightguard vital bleaching: how safe is it? Quintessence Int. 1991;22(7):515-523.
20. Haywood VB. Bleaching of vital and nonvital teeth. Curr Opin Dent. 1992;2:142-149.
21. Haywood VB. The Food and Drug Administration and its influence on home bleaching. Curr Opin Cosmet Dent. 1993;3:12-8.
22. Haywood VB. Considerations and variations of dentist-prescribed, home-applied vital tooth-bleaching techniques. Compend Contin Educ Dent. 1994;17(suppl):S616-S621.
23. Nathanson D, Parra C. Bleaching vital teeth: a review and clinical study. Compend Contin Educ Dent. 1987;8(7):490-497.
24. Kugel G, Perry RD, Hoang E, Scherer W. Effective tooth bleaching in 5 days: using a combined in-office and at-home bleaching system. Compend Contin Educ Dent. 1997;18(4):378-383.
25. Kugel G, Perry R, Papathanasiou A, Kastali S: Combined in-office and at-home bleaching system: an evaluation [abstract]. J Dent Res. 1998;77:957. Abstract 2604.
26. Deliperi S, Bardwell DN, Papathanasiou A. Clinical evaluation of a combined in-office and take-home bleaching system. J Am Dent Assoc. 2004;135(5):628-634.
27. Bowles WH, Ugwuneri Z. Pulp chamber penetration by hydrogen peroxide following vital bleaching procedures. J Endod. 1987;13(8):375-377.
28. Bowles WH, Thompson LR. Vital bleaching: the effects of heat and hydrogen peroxide on pulpal enzymes. J Endod. 1986;12(3):108-112.
29. Fuss Z, Szajkis S, Tagger M. Tubular permeability to calcium hydroxide and to bleaching agents. J Endod. 1989;15(8):362-364.
30. Féliz-Matos L, Hernández LM, Abreu N. Dental bleaching techniques; hydrogen-carbamide peroxides and light sources for activation, an update. Mini review article. Open Dent J. 2015;8:264-268.
31. Gökay O, Tunçbilek M, Ertan R. Penetration of the pulp chamber by carbamide peroxide bleaching agents on teeth restored with a composite resin. J Oral Rehab. 2000;27(5)428-431.
32. American Dental Association. Statement on the Safety and Effectiveness of Tooth Whitening Products. April 2012. https://www.ada.org/en/about-the-ada/ada-positions-policies-and-statements/tooth-whitening-safety-and-effectiveness. Accessed May 4, 2018.
33. McCaslin AJ, Haywood VB, Potter BJ, et al. Assessing dentin color changes from nightguard vital bleaching. J Am Dent Assoc. 1999;130(10):1485-1490.
34. Blankenau R, Goldstein RE, Haywood VB. The current status of vital tooth whitening techniques. Compend Contin Educ Dent. 1999;20(8):781-788.
35. Schuster A, Shwachman H. The tetracyclines; applied pharmacology. Pediatr Clin North Am. 1956;3:295-303.
36. Christensen GJ. Bleaching vital tetracycline stained teeth. Quintessence Int Dent Dig.1978;9(6):13-19.
37. Mello HS. The mechanism of tetracycline staining in primary and permanent teeth. J Dent Child. 1967;34(6):478-487.
38. Stewart RE. Pediatric Dentistry. St Louis, MO: Mosby; 1982:87.
39. Swift EJ, Jr. A method for bleaching discolored vital teeth. Quintessence Int. 1988;19(9):607-612.
40. Shafer WG, Hine MK, Levy BM. A Textbook of Oral Pathology. 3rd ed. Philadelphia, PA: Saunders; 1974.
41. Goldstein RE, Garber DA. Complete Dental Bleaching. Chicago, IL: Quintessence; 1995.
42. Jordan RE, Boksman L. Conservative vital bleaching treatment of discolored dentition. Compend Contin Educ Dent.1984;5(10):803-807.
43. Faunce F. Management of discolored teeth. Dent Clin North Am. 1983;27(4):657-670.
44. Nathanson D. Vital tooth bleaching: sensitivity and pulpal considerations. J Am Dent Assoc. 1997;128(suppl):41S-44S.
45. Kugel G, Kastali S. Nontray whitening. Compend Contin Educ Dent. 2000;21(6):524-528.
46. Javaheri DS, Janis JN. The efficacy of reservoirs in bleaching trays. Oper Dent. 2000;25(3):149-151.
47. Price RB, Sedarous M, Hiltz GS. The pH of tooth-whitening products. J Can Dent Assoc. 2000;66(8):421-426.
48. MacKay M, Perry R, Swift E, et al. Effects of the two home bleaching systems on enamel surfaces [abstract]. J Dent Res. 1997;76(spec iss). Abstract 1405.
49. Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations-a systematic review. Dent Mater. 2004;20(9):852-861.
50. Li Y. Toxicological considerations of tooth bleaching using peroxide-containing agents. J Am Dent Assoc. 1997;128(suppl):31S-36S.
51. Dahl JE, Becher R. Acute toxicity of carbamide peroxide and a commercially available tooth-bleaching agent in rats. J Dent Res. 1995;74(2):710-714.
52. Schulte J, Morrissette D, Gasior E, Czajewski M. Clinical changes in the gingiva as a result of at-home bleaching. Compend Contin Educ Dent. 1993;14(11);1362-1371.
53. Haywood VB, Leonard RH, Nelson CF, Brunson WD. Effectiveness, side effects and long-term status of nightguard vital bleaching. J Am Dent Assoc. 1994;125(9):1219-1226.
54. Haywood VB, Caughman FW, Frazier KB, Myers ML. Tray delivery of potassium nitrate-fluoride to reduce bleaching sensitivity. Quintessence Int. 2001;32(2);105-109.
55. Jorgensen MG, Carroll WB. Incidence of tooth sensitivity after home whitening treatment. J Am Dent Assoc. 2002;133(8);1076-1082.
56. Pohjola RM, Browning WD, Hackman ST, et al. Sensitivity and tooth whitening agents. J Esthet Restor Dent. 2002;14(2);85-91.
57. Leonard RH Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997;28(8);527-534.
58. Wang Y, Gao J, Jiang T, et al. Evaluation of the efficacy of potassium nitrate and sodium fluoride as desensitizing agents during tooth bleaching treatment-a systematic review and meta-analysis. J Dent. 2015;43(8):913-923.
59. Carey CM. Tooth whitening: what we now know. J Evid Based Dent Pract. 2014;14(suppl):70-76.
60. Domínguez A, García JA, Costela A, Gómez C. Influence of the light source and bleaching gel on the efficacy of the tooth whitening process. Photomed Laser Surg. 2011;29(1):53-59.
61. Garber DA. Dentist-monitored bleaching: a discussion of combination and laser bleaching. J Am Den Assoc. 1997;128(suppl):26S-30S.
62. Kugel G, Aboushala A, Sharma S, et al. Maintenance of whitening with a power toothbrush after bleaching treatment. Compend Contin Educ Dent. 2004;25(2):119-131.
63. Donly KJ, Donly AS, Baharloo L, et al. Tooth whitening in children. Compend Contin Educ Dent. 2002;23(1A):22-28.
64. Joiner A. Whitening toothpastes: a review of the literature. J Dent. 2010;38(suppl 2):e17-e24.
65. Brooks JK, Bashirelahi N, Reynolds MA. Charcoal and charcoal-based dentifrices: a literature review. J Am Dent Assoc. 2017;148(9):661-670.
66. Kugel G, Kastali S. Tooth-whitening efficacy and safety: a randomized and controlled clinical trial. Compend Contin Educ Dent. 2000;29(suppl):S16-S21.
67. Kugel G. Over-the-counter tooth-whitening systems. Compend Contin Educ Dent. 2003;24(4A):376-382.
68. Price RB, Loney RW, Doyle MG, Moulding MB. An evaluation of a technique to remove stains from teeth using microabrasion. J Am Dent Assoc. 2003;134(8):1066-1071.
69. Wong FS, Winter GB. Effectiveness of microabrasion technique for improvement of dental aesthetics. Br Dent J. 2002;193(3):155-158.
70. Croll TP. Enamel microabrasion for removal of superficial dysmineralization and decalcification defects. J Am Dent Assoc. 1990;120(4):411-415.
71. Donly KJ, Croll T.Enamel microabrasion for removal of superficial coloration and surface texture defects. In: Perdigão J, ed. Tooth Whitening. Switzerland: Springer International; 2016.
72. Sundfeld RH, Croll TP, Briso AL, et al. Considerations about enamel microabrasion after 18 years. Am J Dent. 2007;20(2):67-72.