You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Implants have a lengthy history in dentistry, dating back to the Mayan population of 600 AD.1 The clinical applications have certainly changed over the years, as have the integrity of the implants and the degree of osseointegration. Although the techniques and materials have drastically improved, the goal of innovative treatment for patients has remained the same: successful osseointegration and restoration. Currently, research supports a greater than 95% success rate for osseointegrated implants.2 With the many advances and superb success rate, implants are quickly becoming the preferred treatment for edentulous areas of the mouth.
Among the many advancements in implant dentistry are the materials used, not only in the implants themselves but in the restorative components. High esthetics are being combined with many avenues of achieving excellent results that meet patients’ expectations. In addition, the methods of gathering necessary data have also improved. Technological progress has enabled a completely digital workflow, from diagnosis to final restoration.
Case Presentation
The patient was a 54-year-old woman who was fairly healthy. She had initially presented with pain associated with her lower right quadrant that was spontaneous and severe. Tooth No. 31 was deemed as fractured and nonrestorable and, therefore, was extracted. The pain only subsided for a few days following extraction. The patient then visited an endodontist to evaluate teeth Nos. 29 and 30, which were vital at that time. She was ultimately sent to an oral surgeon for evaluation.
The oral surgeon’s assessment did not yield a specific diagnosis. Whether the pain was due to a bone (osteomyelitis) infection or tooth (pulpitis/acute apical periodontitis) infection was unclear. The patient was placed on a regimen of antibiotics. Following several days of unresolved pain and concurrent swelling, she was hospitalized and put on intravenous antibiotics. In the hospital, a gallium scan was performed and specific specimens were sent for culture and sensitivity. The results were inconclusive.
The infection was shortly thereafter determined to be acute osteomyelitis, based on the increased swelling and the fact that both teeth were vital 2 days prior. Both chronic (>1 month duration) and acute osteomyelitis (<1 month duration) are commonly caused by the pathogenic bacteria Staphylococcus aureus in patients of this age; however, a number of bacteria can be associated in the oral cavity. It is also interesting that although the incidence of osteomyelitis is on the rise, maxillofacial (mandibular) osteomyelitis is still an uncommon condition. Typical features of osteomyelitis are pain and paresthesia. Many cases are also associated with the diabetic population, although this patient had no such history. It is, however, rare that the mandible is involved.3-5
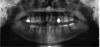
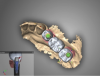
Considering the diagnosis and the options, the surgeon and patient ultimately decided that extraction of teeth Nos. 29 and 30 was the best option at this point. Because these two teeth were now nonvital, the most plausible explanation for the condition was spontaneous necrosis due to the acute osteomyelitis. At the time of surgery, minimal loose bone between the sockets of teeth Nos. 30 and 31 was removed, but there was otherwise no bone removal (Figure 1). This was fortunate, because aggressive bone debridement is often necessary with osteomyelitis.
Treatment Planning
The patient now presented with teeth Nos. 29 through 31 missing (No. 32 had been missing prior to initial presentation), and she definitely wanted to replace them. She also said she did not want anything removable. With no distal extension, a tooth-borne fixed partial denture was not a viable treatment option. The only option the author felt was available was to replace the edentulous area with implants.
The edentulous area could have been partially restored by placing one implant in the No. 30 site and creating a tooth (No. 28)-to-implant (No. 30)–supported prosthesis (TISP). Although this was a possibility, the only true advantage to the patient would have been cost. The recent literature supports that this is a viable option, and that previous concerns with TISPs may be overstated.6,7
A second option was to replace all three missing teeth with individual implants. This treatment would have been hygienic to the patient and, in the event of an implant and/or component failure, could be less complicated to resolve the issue. The challenge to performing this treatment is that placement is critical in order to maintain esthetics and enable an appropriate tooth size. The placement distance between the implants for optimum longevity must also be evaluated. Additionally, this is the least cost-effective solution.
The final option, which was deemed the best alternative for this patient, was an implant-borne fixed partial denture. This was the most sensible option, considering the presentation. The implants were to be placed approximately at the No. 29 and 31 sites. This option was not only a viable solution but also a cost-effective one.
The Process
Following the extractions, the patient began the surgical phase of treatment. Through discussions between the surgeon and general dentist, it was determined that two implants would be placed. To do this, they needed to properly assess the alveolar ridge in terms of height, width, and bone quality. Although periapical and panoramic radiographs have their merits in implant dentistry, they can also present inaccurate representations. Periapical radiographs are excellent for anatomy, pathology, and bone height; however, they are just 2-dimensional images.8 Panoramic radiographs are also valuable for locating pathology and they present a broader image of the jaws and anatomy, but they are also 2-dimensional and prone to distortion.9
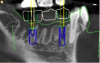
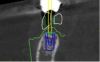
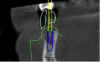
The most accurate representation for imaging in dentistry is cone-beam computed tomography (CBCT). This modality has been designed for implant dentistry. The CBCT scan offers a volumetric assessment of the bone in a 3-dimensional format. By providing the volumetric analysis, use of CBCT has led to reduced implant failures.10 Furthermore, the American Academy of Oral and Maxillofacial Radiology recommends some form of cross-sectional diagnostic imaging for implant placement.11 In the present case, CBCT imaging was utilized for treatment planning.
In addition to the CBCT, impressions were taken of both arches. The impressions were poured in stone, then converted to a digital image using a digital scanner in the laboratory.
Co-diagnostic software was also used. While this type of software is available to practitioners from multiple manufacturers, in this case the laboratory provided the software. Co-diagnostic software allows for a prosthetically driven implant placement. In other words, the restorative outcome is taken into account prior to planning the placement of the implant. The software enables the team to incorporate the scanned image of the arch and the CBCT data to plan the surgery and fabricate a surgical stent, if so desired.12
This software allows accurate visualization of anatomic structures that are to be avoided during placement. This was especially important in this case because of the close proximity of the inferior alveolar nerve to the proposed implant sites. This planning has been found to lead to optimal results and allows the surgeon to place implants safely and efficiently.13-15
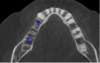
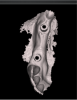
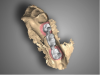
Once the scan and CBCT were loaded into the software, the respective files were merged to create a new image (Figure 2 through Figure 5). The data was then analyzed. The available software has vast libraries, some with up to 3,000 implant components from which to choose, from various manufacturers.12 The appropriate anterior-posterior position was determined, followed by the proper diameter and length of the implant bodies. This was all determined with the restoration in mind. The data was then used to fabricate a guide for the surgeon to use (Figure 6). These decisions were all made collectively by the team.
Following the evaluation and planning phase, the patient was ready for surgery. The surgeon used the guide generated by the software, which can also produce a sequential protocol for the surgeon to utilize throughout the surgery. Employing the capabilities of the software, the surgery was efficient and without incident. In certain instances, the implants can be immediately loaded at placement. The key factor for initial loading is implant stability, which is 35 Ncm.16 Due to the patient’s previous history of osteomyelitis, and to achieve optimal healing, the sites were not immediately loaded with a temporary prosthesis.
After an appropriate healing time of 9 months, the patient returned to the oral surgeon’s office for uncovering, verification of implant stability, and placement of healing caps. The healing time was prolonged due to the patient’s history. Osseointegration is a complicated process dependent upon various factors and determined by several methods.17 The literature does not seem to pinpoint an exact timeline for osseointegration, but for the practitioners in this case the usual waiting time is 3 to 4 months.
Restorative Phase
The final phase of this process, the restorative phase, employs the restorative dentist and the laboratory. At the initial appointment, an impression was taken to begin fabrication of the prosthesis. The author’s office has utilized a digital scanner for all restorative impressioning since late 2012. The laboratory workflow is also digital. Studies indicate that digitally manufactured restorations are as accurate, if not more accurate, than traditional impressions.18-20 In addition, fit has been found to be better and chairside adjustments minimized by using a scanner for restorative implant impressions.21 In lieu of a transfer coping, a scan body is placed at each of the sites to relay the position of the implants to the laboratory. It is extremely important to contact the manufacturer and the lab prior to scanning the area. Different implant systems and software have different scan bodies. In this case, Straumann CARES® (Straumann, www.straumann.com) scan bodies were used (Figure 7 and Figure 8).
The scan was then sent electronically to the laboratory, which incorporated the scan into its software, of which several versions are available. Once the data is imported and the abutment interface of the implant body is determined, abutment design begins.
The final restoration must be taken into account for abutment design. Various choices present. The restorative choice was a screw-retained prosthesis. Retrievability is critical for a 3-unit fixed partial denture in case any of the components fail. Screw-retained restorations provide this flexibility.22 In addition, their usage alleviates the possibility of peri-implantitis associated with cement-retained restorations. Although a cement line can be controlled with a custom abutment, any residual cement can be detrimental to periodontal health. Such effects include tissue damage, bone loss, and/or chronic inflammation.23
After it was decided that a screw-retained restoration would be used, abutment material and design choices needed to be made. Due to the system being used and the availability of authentic components, three abutment choices were available24: titanium; zirconia; and ti-base. Each abutment choice had pros and cons associated with them. While a titanium abutment offers high strength and a zirconia abutment provides good esthetics, in many clinical cases both strength and esthetics are desired. For this case, a ti-base abutment (Variobase™, Straumann) was chosen. It consists of a titanium abutment and a coping, which are fused together as one piece. The height of the abutment is only 3.5 mm, and the width is 2.8 mm to 3.3 mm. The abutment also has four cams for engagement and bonding of the coping.24 The design allows for flexibility in the design of the restoration and coping.
For the restoration itself, a zirconia substructure was used with porcelain veneered over the top of the restoration. This type of restoration is a suitable replacement as compared to a conventional porcelain-fused-to-metal (PFM) restoration. Although the physical properties of the zirconia substrate are improved, the porcelain veneer requires considerable attention to be successful.25,26 In this case, the author and the laboratory prefer the esthetics of the veneered porcelain over a full-zirconia restoration or a conventional PFM restoration. Another factor in this decision was the strength of the restoration and its effect on the opposing dentition. The veneering porcelain to be used, VITA VM®9 (VITA, www.vitanorthamerica.com), has a “wear kindness” to opposing dentition. It also has a reduced particle size and excellent polishability as compared to other veneering porcelains.25
The copings for the implant abutments were designed using the laboratory design software. Initial analysis of the design raised the question of a screw-retained or cemented restoration. As evident in Figure 9 and Figure 10, the access hole on tooth No. 31 was located on the distobuccal cusp. Through communication, the decision was made to proceed, as a screw-retained restoration was desired. Following approval, the laboratory proceeded with the design and production. The framework was fabricated using a highly esthetic and “more translucent” zirconia.
Because the entire laboratory process was digital until layering of the porcelain, the SLA (stereolithographic) resin model was fabricated by the model manufacturer concurrently. One of the benefits of digital dentistry is that the case can be designed independently of model fabrication through the use of digitally defined parameters. The model was received, and the digitally manufactured copings/frameworks were luted to the titanium-base abutments using a resin cement. To finish the restoration, the case was veneered with VITA VM9 porcelain.
Delivery
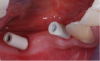
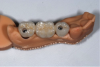
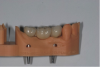
Figure 11 and Figure 12 depict the presentation of the model and restoration for delivery. The case was tried in with minimal adjustments, as is typical with most digital cases.20 One consideration at seating was the gingival collar at tooth No. 31 on the buccal. At initial seat, the tissue was noted to be recessed (Figure 13). The author was left with the options of seating temporarily or retuning to the laboratory to address the affected area. It was decided to seat temporarily and allow the tissue to respond. After 3 weeks the patient returned and the tissue had adapted very well. The decision was made to seat more permanently.
The temporary material was removed, and the access holes were cleaned with a cotton pellet and isopropyl alcohol. The screw torque was verified to be 35 Ncm (minimum 15 Ncm), as dictated by the manufacturer.24 It is critically important to verify the torque values prior to torqueing the abutment, as each implant and abutment manufacturer has a recommended torque value.
Following the torqueing of the implant, the author used a “sandwiching technique” to ensure esthetics of the access hole. Although this is not necessary, patients seem to appreciate the attention to detail and the highly esthetic result. The technique is as follows: (1) place teflon tape directly over the screw; (2) etch the internal surface to the external margin of the access hole; (3) apply universal bonding agent to access up to occlusal margin, then cure; (4) bulk fill composite (for deep access holes) to within 2 mm of access hole, then cure; (5) apply opaqueing resin to 1 mm below the access hole; (6) completely cover the access hole with nanocomposite; (7) polish.
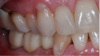
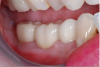
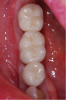
The final restoration is shown in Figure 14 and Figure 15. The patient was extremely pleased with both the result and the process.
Conclusion
In dentistry today, restoring a partially edentulous arch can be very predictable and rewarding; it can also be challenging and confusing. Collaborating as a team is vital to achieve ideal results and eliminate potential errors. Although digital methods have benefited everyday practice in many ways, communication among the dental team is still necessary. Dental practices should consider the prosthetically driven and patient-centered approach discussed in this case an option. Challenging cases require proper preparation and planning. With appropriate communication, high-quality materials, and effective methods, an impeccable workflow and predictable result can be achieved.
ACKNOWLEDGMENTS
The author would like to acknowledge the oral surgeon in this case, David E. Parmer, DDS, MD, of Fort Worth, Texas. Laboratory work was performed by Grady Crosslin, CDT, of Crosslin’s Creative Ceramics in Weatherford, Texas.
ABOUT THE AUTHOR
Chad C. Duplantis, DDS
Private Practice, Fort Worth, Texas
REFERENCES
1. Ring ME, ed. Dentistry: An Illustrated History. 2nd ed. St. Louis, MO: Abradale Press, Mosby; 1985.
2. Setzer FC, Kim S. Comparison of long-term survival of implants and endodontically treated teeth. J Dent Res. 2014;93(1):19-26.
3. Hatzenbuehler J, Pulling T. Diagnosis and management of osteomyelitis. Am Fam Physician. 2011;84(9):1027-1033.
4. Koorbusch GF, Deatherage JR, Curé JK. How can we diagnose and treat osteomyelitis of the jaws as early as possible? Oral Maxillofac Surg Clin North Am. 2011;23(4):557-567.
5. Patel V, Harwood A, McGurk M. Osteomyelitis presenting in two patients: a challenging disease to manage. Br Dent J. 2010;209(8):393-396.
6. Greenstein G, Cavallaro J, Smith R, Tarnow D. Connecting teeth to implants: a critical review of the literature and presentation of practical guidelines. Compend Contin Educ Dent. 2009;30(7):440-453.
7. Shenoy VK, Rodrigue SJ, Prashanti E, Saldanha SJR. Tooth implant supported prosthesis: a literature review. J Interdisciplinary Dentistry. 2013;3(3):143-150.
8. Kumar NA, Agrawal G, Agrawal A, et al. Journey from 2D to 3D: implant imaging a review. Int J Contemporary Dental and Medical Reviews. 2014. Article ID 091114:1-5.
9. Nagarajan, A, Perumalsamy R, Thyagarajan R, Namasivayam A. Diagnostic imaging for dental implant therapy. J Clin Imaging Sci. 2014;4(suppl 2):4.
10. Alamri HM, Sadrameli M, Alshalhoob M, et al. Applications of CBCT in dental practice: a review of the literature. Gen Dent. 2012;60(5):390-400.
11. Tyndall DA, Price JB, Tetradis S, et al; American Academy of Oral and Maxillofacial Radiology. Positional statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(6):817-826.
12. coDiagnostiX™. Dental Wings website. www.codiagnostix.com. Accessed April 13, 2017.
13. Beretta M, Poli PP, Maiorana C. Accuracy of computer-aided template-guided oral implant placement: a prospective clinical study. J Periodontal Implant Sci. 2014;44(4):184-193.
14. Ozan O, Turkyilmaz I, Ersoy AE, et.al. Clinical accuracy of 3 different types of computed tomography-derived stereolithographic surgical guides in implant placement. J Oral Maxillofac Surg. 2009;67(2):394-401.
15. Brief J, Edinger D, Hassfeld S, Eggers G. Accuracy of image-guided implantology. Clin Oral Implants Res. 2005;16(4):495-501.
16. Ostman PO, Hellman M, Sennerby L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin Implant Dent Relat Res. 2005;7 suppl:S60-S69.
17. Parithimarkalaignan S, Padmanabhan TV. Osseointegration: an update. J Indian Prosthodont Soc. 2013;13(1):2-6.
18. Ng J, Ruse D, Wyatt C. A comparison of the marginal fit of crowns fabricated with digital and conventional methods. J Prosthet Dent. 2014;112(3):555-560.
19. Vennerstrom M, Fakhary M, Von Steyern PV. The fit of crowns produced using digital impression systems. Swed Dent J. 2014;38(3):101-110.
20. Pradies G, Zarauz C, Valverde A, et al. Clinical evaluation comparing the fit of all-ceramic crowns obtained from silicone and digital intraoral impressions based on wavefront sampling technology. J Dent. 2015;43(2):201-208.
21. Lee CY, Wong N, Ganz SD, et al. Use of an intraoral laser scanner during the prosthetic phase of implant dentistry: a pilot study. J Oral Implantol. 2015;41(4):126-132.
22. Gervais MJ, Wilson PR. A rationale for retrievability of fixed, implant-supported prostheses: a complication-based analysis. Int J Prosthodont. 2007;20(1):13-24.
23. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications with implants and implant prostheses. J Prosthet Dent. 2003;90(2):121-132.
24. Basic information on the prosthetic procedures. Straumann® Bone Level Implant Line. Straumann website. http://www.straumann.us/dam/internet/straumann_us/resources/guidemanual/handling-instructions/en/uslit-232-bone-level-prosthetic-procedures-manual/USLIT232.pdf. Accessed April 13, 2017.
25. McLaren EA, Giordano RA II. Zirconia-based ceramics: material properties, esthetics, and layering techniques of a new veneering porcelain, VM9. Quintessence Dent Technol. Jan 2005;28:99-111.
26. Giordano R II. Zirconia: A proven, durable ceramic for esthetic restorations. Compend Contin Educ Dent. 2012;33(1):46-49.