You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Vital pulp tissue supports the long-term function of a tooth by contributing to the production of secondary dentin, peritubular dentin, and reparative dentin when biologic and pathologic stimuli are introduced. Pulp tissue moistens the dentin, helping to ensure its resistance and toughness, and otherwise enables teeth to withstand mastication forces.1 Therefore, restorative treatments that preserve pulp vitality—such as those incorporating vital pulp capping—are ideal.1,2
When a proper biologic seal is established and maintained to prevent infiltration of contaminants (eg, bacteria), dental pulp can inherently heal itself through cellular differentiation and bridge formation.1,2 Unfortunately, bacterial microleakage into the pulp via dentinal tubules, as well as procedures performed without proper isolation and/or properly sealing the entire dentin interface during restoration, can result in pulp necrosis and treatment failure.1-4 Ensuring hemostatis is significantly important to the success of pulp-capping procedures (Figure 1 through Figure 5), as is the ability of pulp-capping materials to prevent bacterial microleakage from penetrating through the dentinal tubules and into the pulp.1,2,4,5
Performing successful pulp-capping procedures is predicated on many factors, including establishing thorough hemostasis and selecting appropriate pulp-capping materials. Such materials must be able to relieve clinicians of the challenges they face and not demonstrate characteristics such as cytotoxicity, poor handling properties, low initial mechanical strength, long setting times, and degradation over time.6
CHALLENGES WITH TRADITIONAL PULP-CAPPING MATERIALS
For example, modern dental adhesives that contain hydroxyethyl methacrylate (HEMA) and which are commonly used in pulp-capping procedures have been found to be cytotoxic against an established cell line.7 In fact, in one investigation designed to test cellular toxicity of modern dentin adhesives, the respective cell densities and number of normal, altered, and dead cells were determined and compared with control cell cultures. Statistical analysis of the data showed that all materials tested caused cytotoxic effects, but there were statistically significant differences between the cytotoxicity of the products tested, suggesting some were less cytotoxic than others.8
The cytotoxicity of HEMA in relation to mitochondrial fibroblasts, in particular, has also been studied. By measuring succinate dehydrogenase activity, higher concentrations of HEMA were shown to greatly reduce the health of mitochondria, which is not conducive to pulp capping. It was concluded that the risk of acute cytotoxicity to HEMA through dentin was probably low, but that decreased dentin thickness, lack of polymerization, or extended exposure times might increase risk significantly.9
Therefore, dental product manufacturers have demonstrated significant interest in developing materials that are biocompatible and bioactive, promote dentin bridge formation, and are non-cytotoxic.10 These have included bioactive molecules which, when initially investigated, demonstrated that the formation of homogeneous and mineralized reparative dentin could be induced by adding bioactive molecules to pulp-capping protocol.11 The mechanisms at play in this mineralization process involved the recruitment of cells that subsequently differentiated into osteoblast-like cells, ultimately producing a mineralized extracellular matrix.11,12
It was later determined that direct pulp capping with materials like calcium hydroxide—or implanting bioactive molecules in the pulp—may induce reparative dentin formation, as well as coronal or radicular pulp mineralization.13 However, formation of a dentin bridge, pulp obliteration, or total mineralization of the root canal was found to be dependent upon the type of bioactive molecule used, as well as the condition of the underlying pulp.12-14
Today, a variety of bioactive dental restorative materials are available that promote remineralization and inhibit tooth demineralization. Containing fluoride and/or calcium, these materials range from bonding agents to resin composites, resin cements to sealants, and glass-ionomer cements to pulp-capping materials.15 Calcium release and the ability to sustain a positive alkalinity has been found necessary for ionic exchange, calcium deposition, and the development of new dentin so that a dental bridge can be formed.16,17
Although materials containing calcium hydroxide have been used in pulp-capping techniques and demonstrated success,1 debates regarding the compound’s suitability for this application have focused on its disintegration and breakdown over time (Figure 6 through Figure 10), as well as its tendency to pull from cavity walls during polymerization.1,18,19 Additionally, the ability of calcium hydroxide to promote sclerotic and reparative dentin formation, dentinogenesis, and dentin bridge formation has been questioned.20
BIOACTIVE CALCIUM-SILICATE PULP-CAPPING MATERIALS
More recently researched bioactive restorative resin composites are those containing calcium-silicate, which, in a simulated oral environment, were shown not to be inert.21 Such a material that could form apatite on the surface would be beneficial for gap closure at resin–dentin interfaces, generating better bond strengths over time and preventing bond degradation.21 Evaluations of microtensile bond strength and microhardness demonstrated that calcium-silicate–filled composite resins performed better than etch-and-rinse adhesives and produced a therapeutic and protective effect on the micromechanical properties of mineral-depleted resin–dentin interfaces.22 The implication is that calcium-silicate dental restorative materials can create a more biomimetic restoration, mimic the physical characteristics of tooth structure, and stabilize and protect dental hard tissues.22
Hydraulic calcium-silicate cements, otherwise known as mineral trioxide aggregate cements, are composed mainly based on di- and tri-calcium silicate, Al- and Fe-silicate (eg, Portland cement).23 Originally introduced in the 1990s, hydraulic calcium-silicate cements have traditionally demonstrated biocompatibility, acceptable mechanical properties, and setting and sealing abilities in moist and blood-contaminated environments.23 They also release more calcium than conventional calcium-hydroxide pulp-capping materials.24 However, limitations of early product offerings included low radiopacity, poor handling qualities, and long setting times.23
In recent years, hydraulic calcium-silicate cements have been enhanced with setting modulators, radiopacifying agents, and other additives to the extent that their biological and translational characteristics lend to their applications in root-end filling, pulp capping, scaffolds for pulp regeneration, and as root canal sealers.23 Essentially, these new hydraulic calcium-silicate cements are biointeractive (ie, ion-releasing), bioactive (ie, apatite-forming), and functional.24 They upregulate the differentiation of osteoblasts, fibroblasts, cementoblasts, odontoblasts, pulp cells, and stem cells. Additionally, because they can induce the formation of a calcium-phosphate/apatite coating when immersed in biological fluids, their ability to promote calcium-phosphate deposits implies their potential application in dentin remineralization and tissue regeneration.23 The high rate of calcium release and rapid apatite formation demonstrated by hydraulic calcium-silicate cements further supports their use as scaffolds to induce new dentin bridge formation, as well as clinical healing.24
Studies of a commercially available light-curable pulp-capping material composed of resin and calcium silicate have shown that the material demonstrates high calcium-releasing abilities, low solubility, and durable bond strengths and is able to alkalinize surrounding fluids.25,26 These properties, in addition to the material’s low suppression of mitochondrial health (ie, it demonstrates low cytotoxicity), are highly beneficial in direct pulp-capping treatments.27 Its calcium release stimulates hydroxyapatite, secondary dentin bridge formation, and creation of a protective seal, while alkalinization promotes healing and apatite formation.25,28
CASE PRESENTATION
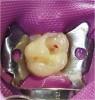
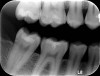
Radiographic examination of a patient’s tooth revealed an abnormality that was subsequently evaluated using a laser caries detection aid. A reading of 68 was obtained. A large tubular dentin defect in the mesial fossa was identified; therefore, the decision was made to treat the tooth (Figure 11).
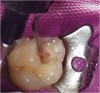
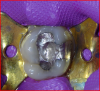
The tooth was prepared, with care taken not to expose the pulp or remove all of the large tubular dentin, but only those aspects that were soft. This would promote remineralization via application of the aforementioned apatite-stimulating, light-cured, resin-modified calcium-silicate material (TheraCal®, Bisco, www.bisco. com). The enamel was etched for 30 seconds, after which the dentin was etched for 3 to 5 seconds (Figure 12).
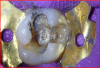
The calcium-releasing resin-modified calcium-silicate material was placed on the affected dentin for remineralization (Figure 13). The material was light-cured for 20 seconds with at least 500 mW/cm2 (Figure 14).
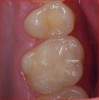
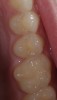
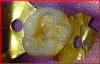
To begin the restorative process, a self-etching adhesive was placed, creating a glossy appearance to the calcium-silicate liner, and the adhesive was light-cured (Figure 15 and Figure 16). A nanohybrid restorative material was then placed to replace the enamel (Figure 17). Upon completion of the restoration, the rubber dam was removed, the occlusion verified (Figure 18), and the restoration polished.
CONCLUSION
When bioactive restorative materials are used for vital pulp-capping protocol, beneficial outcomes result from the induction of physical and chemical processes that mimic those of natural teeth. Although the success of pulp-capping procedures is predicated on many factors—such as ensuring hemostasis and proper isolation—selecting a material that is biocompatible and bioactive, promotes dentin bridge formation, and is non-cytotoxic can help to stabilize and protect dental hard tissues for the long term.
REFERENCES
1. Stockton LW. Vital pulp capping: a worthwhile procedure. J Can Dent Assoc. 1999;65(6):328-331.
2. Stanley HR. Pulp capping: conserving the dental pulp. Can it be done? Is it worth it? Oral Surg Oral Med Oral Pathol. 1989;68(5):628-639.
3. Cox CF. Biocompatibility of dental materials in the absence of bacterial infection. Oper Dent. 1987;12(4):146-152.
4. Bergenholtz G, Cox CF, Loersche WJ, Syed SA. Bacterial leakage around dental restorations: its effect on the dental pulp. J Oral Pathol. 1982;11(6):439-450.
5. Pashley DH. The effects of acid etching on the pulpodentin complex. Oper Dent. 1992;17(6):229-242.
6. Chang KC, Chang CC, Chen WT, et al. Development of calcium phosphate/sulfate biphasic cement for vital pulp therapy. Dent Mater. 2014;30(12):e362-e370.
7. Koliniotou-Koubia E, Dionysopoulos P, Koulaouzidou EA, et al. In vitro cytotoxicity of six dentin bonding agents. J Oral Rehabil. 2001;28(10):971-975.
8. Szep S, Kunkel A, Ronge K, Heidemann D. Cytotoxicity of modern dentin adhesives—in vitro testing on gingival fibroblasts. J Biomed Mater Res. 2002;63(1):53-60.
9. Bouillaguet S, Wataha JC, Hanks CT, et al. In vitro cytotoxicity and dentin permeability of HEMA. J Endod. 1996;22(5):244-248.
10. Moore AN, Perez SC, Hartgerink JD, et al. Ex vivo modeling of multidomain peptide hydrogels with intact dental pulp. J Dent Res. 2015;94(12):1773-1781.
11. Goldberg M, Six N, Decup F, et al. Application of bioactive molecules in pulp-capping situations. Adv Dent Res. 2001;15:91-95.
12. Goldberg M, Six N, Decup F, et al. Bioactive molecules and the future of pulp therapy. Am J Dent. 2003;16(1):66-76.
13. Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15(1):13-27.
14. Oguntebi B, Clark A, Wilson J. Pulp capping with Bioglass