You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The introduction of new materials, products, and their accompanying techniques has changed the manner in which dental implant treatments are performed. Concurrently, they are enabling dentists to increasingly embrace and practice a minimally invasive treatment philosophy. For dental implant treatments—particularly in the esthetic zone—a minimally invasive approach is predicated on thorough diagnosis, thoughtful and comprehensive treatment planning that begins by envisioning the ultimate outcome, and incorporating those materials and procedures that enable predictability with the fewest surgeries, interventions, appointments, and augmentations.1-3
Thoughtful and comprehensive treatment planning involves consideration of all facets of the patient’s condition and what ultimately will be the least amount of dentistry to satisfy their functional, clinical, and esthetic needs and demands.2,3 This requires an assessment of the patient’s underlying bone/tissue health; an evaluation of current esthetic status and how it can be preserved (eg, Are tissue margins symmetrical and at appropriate levels/height? Do soft-tissue contours create a healthy, natural profile?); and potential complicating challenges that may result from any procedures performed (eg, extraction and removable appliance could lead to hard- and soft-tissue collapse, loss of papilla, and loss of ridge width and height).4-6 Because “minimally invasive” is a relative term, the biggest determinants of what the most appropriate minimally invasive approach would be for a given patient are dependent upon treatment decisions and case management.2,5,6
Fortunately, today’s intervention techniques and materials for implant treatments enable dentists to manage the hard and soft tissues at the time of tooth extraction, preserve existing esthetics, and reduce the amount of surgery required for completing the treatment plan. Enabling an overall minimally invasive approach to esthetic implant treatments are flapless extraction, bone/soft-tissue grafting, and implant placement techniques, ridge volume preservation (as opposed to augmentation) procedures, and growth factor/biologic materials.2,7,8
CONSIDERATIONS FOR FLAPLESS TECHNIQUES
Flapless techniques are indicated when a patient presents with good tissue health and contour, volume in the marginal gingiva supported by healthy bone, and overall good tissue volume.1,7 Ideal when the gingival tissue exhibits an esthetic nature, flapless techniques require care to avoid altering tissue contour and quality (ie, creating scar tissue). In some cases, a modified flapless approach (eg, using submarginal incisions) may be necessary in order to maintain the marginal gingival profile.8
For example, even in a case with a complete loss of the buccal plates, the least invasive option may still be a flapless coronal approach combined with a submarginal incision. The incision can be made with a diode laser, including a frenectomy, to enable access for cleaning the apical area with piezosurgery.9 After extraction and debridement, bone material can be packed into place; a small membrane placed over the graft material on the labial aspect; the labial incision closed with a 6-0 chromic gut suture; and a small layer of collagen membrane placed just within the surface tissue present at the coronal aspect.10 After healing, an implant can be placed in a minimally invasive, guided surgical/tunneling approach.8
However, transitioning from a tooth to an implant without damaging the esthetics of the existing tissue can present challenges. In most instances, soft-tissue grafting may be required to thicken the gingival tissue biotype.11 Sites that have had connective tissue grafting demonstrate much more stable marginal tissue levels, particularly at 12 months after restoration.12,13 Although surgery may not be desired, it is oftentimes necessary considering the long-term marginal tissue level stability that is achieved.11
RIDGE PRESERVATION VERSUS AUGMENTATION
The sites in which implants are to be placed may present with radiographic evidence (eg, CBCT) of bone loss or damage that necessitates consideration of ridge and/or volume preservation versus augmentation.14,15 Because tooth roots help maintain tissue height and contour, their removal leads to collapse. Additionally, in the presence of thin bone, extraction initiates bone shrinkage within 6 weeks. Therefore, intervening with grafting (eg, hard- or soft-tissue grafting) at the time of extraction helps to preserve the volume with which a patient presents.14,15
Alternatively, augmentation techniques are associated with staged interventions (ie, delayed grafting). By extracting the tooth and allowing the tissues to heal, different challenges can present when subsequently performing additional surgical procedures to augment the tissues.16 For this reason, anticipating the end results of treatment is necessary to plan how the best outcome can ultimately be achieved.3-5
Areas where augmentation is required due to trauma, changes in tooth position, and/or loss of bone and/or tissue volume and contour in specific regions may require grafting.16-18 Typically, flap procedures for ridge augmentation can be performed to enable hard-tissue augmentation to increase the width of the ridge, and connective tissue grafts (as needed) can be placed to enhance soft-tissue volume.2,7,8,16-18 After bone healing and successful thickening of the tissue, the site can be reentered for implant placement; excess tissue volume can be shaped with a contoured healing abutment and provisional restoration.19 This will allow good preservation of the papilla and maintenance of stable marginal tissue levels.
GROWTH FACTORS, BIOLOGICS, AND rH-PDGF-BB
The use of growth factors in dentistry essentially transforms the healing process following hard- and soft-tissue periodontal procedures, as well as implant surgeries. Whereas typical bone healing with graft materials is osteoconductive (ie, bone emerging only from the lateral edges of a defect), growth factors promote more robust levels of new bone formation through osteoinductive mechanisms (ie, bone growth and enhanced bone metabolism throughout the extraction socket or defect at an equal pace).20-22
Platelet-derived growth factor (PDGF) is approved in the United States by the Food and Drug Administration for periodontal regeneration based on its ability to re-grow bone and tissues around teeth. PDGF works by directly affecting the osteoblastic cells and, secondarily, by raising vascular endothelial growth factor (VEGF).10,23,24 In bone healing, the two most important aspects are wound stability and vascular supply.
PDGF upgrades the vascular supply within the wound indirectly through VEGF, which stimulates new capillary formation therein, and also upgrades bone metabolism.10,11,23,24 In fact, there is an increase in bone metabolism with a surrogate marker of bone turnover and interstitial collagen telopeptide only found in bone.10,18,20 As a result, when treating a large defect with freeze-dried bone and PDGF using a flapless approach, it is possible to take a bone biopsy at 5 months and, under high-power magnification, observe new bone growing through the graft particle.18,20,21
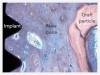
The extent of regeneration that can be achieved with PDGF has been demonstrated in the literature, particularly when researchers took bovine bone block soaked in PDGF and, 4 months later, observed new bone grown over the top of the defect (Figure 1 through Figure 3).22 Without PDGF, there was almost no new bone or soft tissue growing in or around the bovine-bone product.
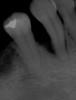
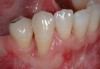
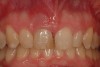
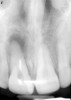
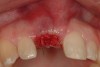
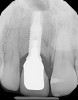
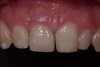
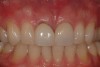
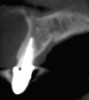
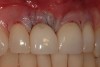
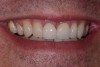
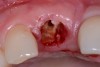
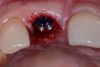
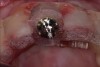
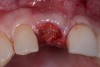
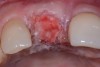
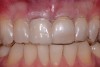
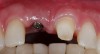
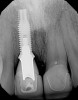
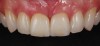
Among the commercially available growth factors today is recombinant human platelet-derived growth factor (rh-PDGF)-BB. Manufactured through recombinant biotechnology, it is a synthetically engineered, pure human protein used in esthetic implant cases to ensure the greatest predictability, even in challenging sites (Figure 4 through Figure 7).21,22 Another commercially available growth factor is bone morphogenetic protein-2 (BMP-2), which essentially has been touted as “doing the work for us.”25 Only a few months after placement—even in cases with a complete lack of buccal plate and loss of palatal bone—almost complete reconstitution of the ridge form has been observed upon reentry.25
IMPLICATIONS FOR PATIENT TREATMENT
The use of growth factor technology, combined with advances in minimally invasive surgical techniques, has changed practices significantly over the past decade. As a result, dentists can modify the manner in which they approach treatments for their patients who require extractions, implants, and corrective bone and soft-tissue procedures—all with less invasiveness but with greater predictability. The following cases demonstrate the manner in which growth factor technology, combined with minimally invasive techniques, has resulted in predictable outcomes in esthetic dental implant treatments.
Case Report 1
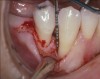
A patient presented with a large periapical lesion, root fracture, and significant loss of buccal bone (Figure 8 and Figure 9). Treatment for this case involved an autogenous bone graft, then implant placement and connective tissue grafting. Recombinant human platelet-derived growth factor-BB was used for ridge preservation and minimally invasive esthetic implant site development with a flapless approach.
The tooth was extracted, and magnification was used while debriding and degranulating the extraction socket. The site was irrigated with a significant amount of sterile water from 10-mL syringes, and the water pressure helped with debridement. Spoon and #4 Gracey curettes were used.
Once degranulated and debrided, the growth factor matrix (eg, freeze-dried bone allograft that was presoaked in rh-PDGF-BB for about 10 minutes) was condensed into the site. The site was then covered with a collagen membrane and sutured.
After healing, the site was evaluated and a high frenum attachment was observed (Figure 10). A frenectomy was performed prior to implant placement, along with a connective tissue graft to thicken the soft tissues.
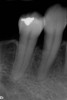
At 8 years post-treatment, implant bone levels were maintained as evidenced radiographically (Figure 11), with stable gingival margins and bone levels at the first thread of the fixture. There was minimal visible damage and scar tissue from the surgical approach to implant placement and tissue grafting to thicken the biotype. Despite the potential challenges, the use of growth factor technology and minimally invasive surgical techniques allowed the esthetic treatment goals for this case to be achieved for the long term (Figure 12).
Case Report 2
A patient presented with a worn crown and post that had been removed and re-cemented several times (Figure 13 and Figure 14). Among the approaches that could have been considered was an extensive procedure to include extraction, implant placement, bone grafting, and soft-tissue grafting simultaneously.
However, by giving consideration to the most appropriate and minimally invasive sequence of care, this led to performing predictable procedures beginning with a tunneling procedure with a small connective tissue graft (Figure 15). Once the connective tissue graft healed (Figure 16), extra soft tissue with a thickened biotype was available. This would enable minimally invasive extraction using piezosurgery—without damaging the soft tissues (Figure 17)—followed by immediate implant placement according to 3D implant treatment planning (Figure 18 through Figure 20).
After implant placement, bone graft material enhanced with growth factor was also placed, which would be protected by the extra tissue height (Figure 21 and 22). An Essex appliance was used for the first 2 weeks (Figure 23), and once the soft tissues healed, the crown on the adjacent tooth could be removed to allow for an interim cantilever prosthesis.
Second-stage surgery involved uncovering the implant and finalizing the prosthetic treatment (Figure 24). The ultimate outcome represented a thickened biotype, healthy bone (Figure 25), and an inconspicuous implant-supported crown restoration (Figure 26).
CONCLUSION
In a minimally invasive approach to esthetic implant treatments, incorporating updated surgical approaches and harnessing the potential of growth factor technology can make treatments predictable and less invasive for patients. By beginning the treatment planning process with a vision of the ultimate outcome and then working backwards, dentists can best understand and plan what will be needed surgically to achieve those results. Flapless techniques, a focus on ridge volume preservation at the time of extraction versus post-healing remodeling, and using recombinant human platelet-derived growth factors allows for the fewest surgical interventions for esthetic implant site development and restoration.
REFERENCES
1. Wang F, Huang W, Zhang Z, et al. Minimally invasive flapless vs. flapped approach for single implant placement: a 2-year randomized controlled clinical trial. Clin Oral Implants Res. 2016 May 19. doi: 10.1111/clr.12875. [Epub ahead of print].
2. Hayashi J, Shin K, Takei HH. Minimally invasive surgical approaches for esthetic implant dentistry: a case report. J Oral Implantol. 2016;42(1):93-97.
3. Dawson PE, Cranham JC. Aesthetics and function: conflict or complement? Dent Today 2007;26(10):80-83.
4. Petropoulou A, Pappa E, Pelekanos S. Esthetic considerations when replacing missing maxillary incisors with implants: a clinical report. J Prosthet Dent. 2013;109(3):140-144.
5. Shah KC, Lum MG. Treatment planning for the single-tooth implant restoration—general considerations and the pretreatment evaluation. J Calif Dent Assoc. 2008;36(11):827-834.
6. Leblebicioglu B, Rawal S, Mariotti A. A review of the functional and esthetic requirements for dental implants. J Am Dent Assoc. 2007;138(3):321-329.
7. Tsoukaki M, Kalpidis CD, Sakellari D, et al. Clinical, radiographic, microbiological, and immunological outcomes of flapped vs. flapped dental implants: a prospective randomized controlled clinical trial. Clin Oral Implants Res. 2013;24(9):969-976.
8. Nevins ML, Camelo M, Nevins M, et al. Minimally invasive alveolar ridge augmentation procedure (tunneling technique) using rhPDGF-BB in combination with three matrices: a case series. Int J Periodontics Restorative Dent. 2009;29(4):371-383.
9. Stübinger S, Stricker A, Berg BI. Piezosurgery in implant dentistry. Clin Cosmet Investig Dent. 2015;7:115-124.
10. Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-2215.
11. Farina V, Zaffe D. Changes in thickness of mucosa adjacent to implants using tissue matrix allograft: a clinical and histologic evaluation. Int J Oral Maxillofac Implants. 2015;30(4):909-917.
12. Grunder U. Crestal ridge width changes when placing implants at the time of tooth extraction with and without soft tissue augmentation after a healing period of 6 months: report of 24 consecutive cases. Int J Periodontics Restorative Dent. 2011;31(1):9-17.
13. Schneider D, Grunder U, Ender A, et al. Volume gain and stability of peri-implant tissue following bone and soft tissue augmentation: 1-year results from a prospective cohort study. Clin Oral Implants Res. 2011;22(1):28-37.
14. Jambhekar S, Kernen F, Bidra AS. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: a systematic review of randomized controlled clinical trials. J Prosthet Dent. 2015;113(5):371-382.
15. Tomlin EM, Nelson SJ, Rossmann JA. Ridge preservation for implant therapy: a review of the literature. Open Dent J. 2014;8:66-76.
16. Doonquah L, Lodenquai R, Mitchell AD. Surgical techniques for augmentation in the horizontally and vertically compromised alveolus. Dent Clin North Am. 2015;59(2):389-407.
17. Aloy-Prósper A, Peñarrocha-Oltra D, Peñarrocha-Diago M, et al. Peri-implant hard and soft tissue stability in implants placed simultaneously versus delayed with intraoral block bone grafts in horizontal defects: a retrospective case series study. Int J Oral Maxillofac Implants 2016;31(1):133-141.
18. Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54(4):420-432.
19. Shor A, Schuler R, Goto Y. Indirect implant-supported fixed provisional restoration in the esthetic zone: fabrication technique and treatment workflow. J Esthet Restor Dent. 2008;20(2):82-95.
20. Cochran DL, Schenk R, Buser D, et al. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J Periodontol. 1999;70(2):139-150.
21. Nevins ML, Reynolds MA. Tissue engineering with recombinant human platelet-derived growth factor BB for implant site development. Compend Contin Educ Dent. 2011;32(2):18-27.
22. Simion M, Rocchietta I, Dellavia C. Three-dimensional ridge augmentation with xenograft and recombinant human platelet-derived growth factor-BB in humans: report of two cases. Int J Periodontics Restorative Dent. 2007;27(2):109-115.
23. Sarment DP, Cooke JW, Miller SE, et al. Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol. 2006;33(2):135-140.
24. Nevins ML, Camelo M, Schupbach P, et al. Human buccal plate extraction socket regeneration with recombinant human platelet-derived growth factor BB or enamel matrix derivative. Int J Periodontics Restorative Dent. 2011;31(5):481-492.
25. Misch CM. The use of recombinant human bone morphogenetic protein-2 for the repair of extraction socket defects: a technical modification and case series report. Int J Oral Maxillofac Implants. 2010;25(6):1246-1252.
EDITOR’S NOTE
Figure 1 through Figure 3 republished with permission of Quintessence Publishing Company Inc, from Simion M, Rocchietta I, Dellavia C. Three-Dimensional Ridge Augmentation with Xenograft and Recombinant Human Platelet-Derived Growth Factor-BB in Humans: Report of Two Cases. Int J Periodontics Restorative Dent. 2007;27(2):109-115; permission conveyed through Copyright Clearance Center, Inc.
Figure 4 through Figure 7 reproduced from J Periodontol. 2013;84(4):456-464. Used with permission from the American Academy of Periodontology.