You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
GLOSSARY
Antimicrobial - destroying or preventing the development of microoganisms; also, an agent with such activity
Bacteremia - introduction of bacteria to the bloodstream
Bacteriostasis - inhibition of bacterial growth without destruction
Crevicular - pertaining to a crevice, particularly the gingival crevice
Cytotoxic - destructive to cells
Embrasure - v-shaped space between the proximal surfaces of adjacent teeth
Fibroblast - cell that develops connective tissue
Galvanic - of or relating to direct-current electricity, especially when produced chemically; also having the effect of an electric shock
Junctional epithelium - cufflike band of stratified squamous epithelium continuous with the sulcular epithelium encircling the tooth providing a seal at the base of the sulcus
Osseointegration - attachment of healthy bone to an implant
Osteolytic - pertaining to the loss of bone
Pellicle - thin coating of salivary materials that are deposited on tooth surfaces
Peri- - prefix: around or surrounding (for example, “perioral” means “surrounding the mouth”)
Pontic - an artificial tooth
Radiolucent - allowing radiation to pass through, presenting as a dark area on a radiograph
Substantivity - a property of certain active ingredients that inhibits growth of bacteria on the skin and other body tissues
In recent years, the demand for dental implants has risen greatly. Osseointegrated dental implants are being placed with increased frequency. It is estimated that approximately 1 million dental implants are placed in the United States annually. Not only have placement techniques improved, but the benefits that implants provide for patients have increased as well. Dental implants improve appearance, confidence, and self-esteem. Implants also preserve remaining teeth, improve a person’s ability to speak and masticate properly, and eliminate the need for full and partial dentures. Because dental implants present a significant financial investment and require long-term maintenance by the patient for a healthy peri-implant environment, the direct impact of oral hygiene maintenance by the patient will determine long-term prognosis and success of the dental implant.
The mucoperiosteal-implant seal is the major factor in determining long-term prognosis. Indigenous oral bacteria attaching to dental implant surfaces can lead to the breakdown of the biological seal surrounding the dental implant. Although the junctional epithelium attachment for dental implants is similar to natural dentition, the connective tissue interface with the dental implant has poor mechanical resistance. The peri-implant disease process resembles periodontitis. However, treatment and maintenance are more complex. The tissues around dental implants react to bacteria similarly to the tissues around natural teeth. In fact, plaque develops more rapidly and in larger amounts around titanium implant abutments than around natural teeth. Therefore, close cooperation and teamwork among dental providers and their patients is essential to the success of dental implant procedures. Many of the current home care treatments for periodontal maintenance of natural teeth also can be used with dental implants, but a better understanding of oral health maintenance by the patient is crucial for the health and longevity of dental implants.
PREVENTATIVE MAINTENANCE
If the titanium oxide layer of the dental implant is disrupted during oral hygiene procedures, the soft tissues may be exposed to titanium metallic ions that can cause potentially cytotoxic reactions, compromising the dental implant. Therefore, detailed instructions by the dental professional should be given initially to the patient and reinforced at each recare appointment to prevent trauma or infection to the sulcus around the implant. The removal of early microbial accumulation on the dental implant surfaces and the elimination of at least 85% of plaque biofilm by the patient are crucial for long-term peri-implant success. The preventive maintenance steps for dental implants involve two distinct aspects: (1) patient self-care, and (2) clinical maintenance procedures.
Patient Self-Care
No single device has been shown to remove plaque from all surfaces of an implant reconstruction. While there are numerous types of brushes, threading systems, flosses, and other oral hygiene devices on the market, the literature substantiates the need to minimize the number of devices prescribed for patient self-care. Patient compliance, an essential aspect of any maintenance program, predominantly depends upon the relative simplicity of a procedure, the time required, and a minimum number of devices being employed.
Studies indicate when multiple oral hygiene devices are prescribed, patients can become discouraged and, as a result, may be less motivated. However, research shows additional plaque inhibition with a combination of tooth brushing, auxiliary aids, and antimicrobial mouthrinses. For this reason, it is important to consider appropriate combinations when making recommendations to individual patients.
Manual and Power Tooth Brushing
Various types of toothbrushes may be used to clean implant superstructures. Exposed facial and lingual areas of the dental implant, its fixed and/or removable prosthesis, and surrounding gingival tissues can be cleaned using a soft, multitufted, nylon toothbrush. There are many different brush handle angles from which to choose. The dental professional should assist the patient in choosing a handle that allows the patient to successfully access all areas of the oral cavity. The modified Bass technique should be used, or a short, horizontal back-and-forth movement can be employed. In the modified Bass technique, the brush is held at a 45-degree angle where the abutment post meets the gingival tissue (Figure 1). Patient tooth brushing techniques often miss cleaning the most lingual aspect of the titanium abutment cylinders, so patients must be instructed to give special attention to the lingual aspects.
Rotary unitufted power brushes, oscillating-rotating brushes, and sonic brushes do not damage polished implant surfaces and also can be safely used to clean the facial, lingual, and interproximal areas of the implant. Many power brushes are equipped with soft interchangeable bristle heads (flattened, rubber-cup-like, short and long pointed in shape). The short and long pointed tips are ideal for reaching proximal areas of the tooth, those areas with wide embrasures, and those areas located beneath the pontic portion of a fixed bridge. The hollowed, rubber cup should be used on the facial and lingual aspects of the implant and adjacent teeth. The brush tip should be dipped in a 0.12% solution of chlorhexidine gluconate. Research associated with the utilization of this solution shows a reduction in certain bacteria by 54% to 97% after 6 months use. The very fine bristles of a rotary unitufted power toothbrush simultaneously debride the implant surface and deliver the antimicrobial solution to the crevicular area. One oral hygiene implant study examined the Rotadent® rotary unitufted power brush (Zila, Inc., rotadent.com) and the Proxabrush® Interdental System (manual interproximal cleaning aids) (Sunstar Americas, Inc., gumbrand.com). Results demonstrated “virtually no change in surface appearance from the original machined implant and its surface irregularities.”
Auxiliary Aids, Antimicrobial Rinses, and Dentrifice
In certain situations, interproximal brushes with small brush heads may be necessary to gain easier access. However, such devices must be plastic-coated because metal can damage or contaminate an implant’s titanium surface.
An interdental brush can be used to massage the gingival tissue around an implant to increase blood flow and enhance the tone of the surrounding gingiva. The patient should be instructed to insert the tip interdentally in an occlusal direction, pressing the side of the tip against the marginal gingiva and applying a gentle rotary motion.
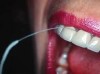
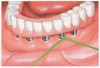
Floss can be designed as a wide band of ribbon with one end designed for use as a threading device that can be threaded around abutments and beneath frameworks. Especially designed for implant care, flosses such as SuperFloss® (Oral-B, oralb.com) (Figure 2) or Postcare® (Sunstar Americas, Inc.) (Figure 3) can be used in conjunction with chlorhexidine gluconate. Used in the manner of a “shoe-shine rag” (ie, a side-to-side motion), the ribbon polishes the back and sides of the post from top to bottom. This cleansing action produces positive results for plaque control around fixtures and abutment cylinders, as well as the cervical aspect. In areas with smaller interfixtural dimension, traditional unwaxed floss may be used with a floss threader.
The oral irrigator is a beneficial adjunct for removing supragingival soft debris around implants. However, caution must be exercised by patients when using this device. Incorrect use and excessive water pressure can damage the junctional epithelium, leading to bacteremia. To prevent these problems, patients must receive instruction to use the lowest water-pressure setting. Furthermore, patients are educated to place the irrigator tip in the interproximal area horizontal to the implant and along its gingival margin to avoid subgingival spray. An oral rinse containing chlorhexidine gluconate or phenolic compounds may be used as an irrigant.
Microbial plaque plays a major role in both adult periodontitis and peri-implantitis. Similar microbial flora are found around the gingival crevices of both adult periodontal disease and failing implants. The regular use of chemotherapeutic agents such as antiseptic mouthrinses may be recommended to the dental implant patient to combat these concerns.
Chlorhexidine gluconate is a safe, nontoxic adjunct to other oral hygiene procedures in the maintenance of dental implants. An American Dental Association-accepted chlorhexidine gluconate mouthrinse can be very effective due to its substantivity (binding activity to the tissues in the oral cavity and on titanium abutment surfaces). Treating implant patients with chlorhexidine gluconate mouthrinses aids in fibroblast attachment to implant surfaces. The acquired pellicle acts as a chemical reservoir source, releasing chlorhexidine gluconate over a prolonged period of time in concentrations sufficient to maintain bacteriostasis.
About 90% of the cultivable bacteria are inhibited for about 5 hours with a 0.12% concentration of chlorhexidine gluconate rinsing for 30 seconds. Because staining of composites often accompanies long-term use of chlorhexidine gluconate rinses, it can be applied with a cotton swab when composite restorations are present. Patients should be advised that chlorhexidine gluconate use also can diminish taste sensation for salty foods. Some of these rinses have no effect on the dental implant surface itself. It may be safe to assume that other antimicrobial agents such as phenolic compounds also produce no surface alteration. As an oral rinse, application is recommended once daily with a chlorhexidine gluconate formulation or twice daily with most over-the-counter therapeutic rinses.
Auxiliary aids such as angled brushes, floss threaders, sulcular brushes, interdental cleaners, flossing aid, and irrigation devices are all alternative secondary mechanical plaque-control aids, but again, limiting the number of devices is important for patient compliance.
Disclosing solutions and tablets are a valuable aid in revealing the presence of plaque to the implant patient. Inspection of disclosed areas assists the patient in identifying areas of plaque retention and provides immediate feedback on the effectiveness of oral hygiene procedures.
Clinical Maintenance Procedures
At each recare visit, the dental professional should perform a clinical assessment of peri-implant soft tissues by examining the color, surface texture, and note any bleeding and inflammation. When probing, the use of a non-metal periodontal probe will not contaminate the titanium surface and is gentle to tissue and safe against dental implant surfaces. Some clinical researchers suggest that periodontal probing be performed at infrequent intervals at one site (the same site each time) with light pressure. As with natural dentition, the dental professional must be careful not to contaminate the implant sulcus with bacteria from a diseased periodontal sulcus. It is recommended that the periodontal probe be dipped in chlorhexidine between measurements to avoid contaminating a healthy site with microflora from a diseased site.
Although 3 mm is considered healthy for natural dentition, probing depths for implants can range from 2.5 mm to 5 mm depending on soft-tissue depth, as the probe goes beyond the sulcus, through the junctional epithelial attachment and connective tissues, placing it closer to the alveolar bone. As a rule, the ideal sulcus depth should be less than 5 mm, as sulcus depths greater than 5 mm to 6 mm have a potential for anaerobic bacteria. Increased probing depths have been correlated with failing implants; 58% of failing implants are characterized by pocket depths greater than 6 mm. The world-renowned Brånemark Group found that an average marginal bone loss of 1.5 mm occurred during the first year of prosthesis connection and an average of 0.1 mm every year thereafter. Any bone loss exceeding these averages should raise concern.
The major difference between gingival attachment to a natural tooth and a dental implant is that the implant surface lacks cementum with connective tissue fiber inserts. Gingivitis most likely progresses to periodontitis around the implant due to the unreliability of the perimucosal seal and the lack of fiber barriers between the implant and the soft tissue of the sulcus.
When examining the implant, the dental professional must chart the presence of plaque and calculus deposits around the implant surfaces. The bacteria responsible for periodontitis are the same for peri-implantitis. These pathogenic bacteria are gram-negative anaerobic bacteria; they include bacteroides forsythus, actinobacillus actinomycetemcomitans, porphyromonas gingivalis, and treponema denticola and have shown to contribute to failing implant sites. After the soft tissue has been examined, the next step is to evaluate mobility of the implants, transmucosal abutments, and prosthetic superstructure. Seventy-eight percent of failing implants have excess mobility. Mastication or lack of tissue stability at the junction of the dental implant and connective tissue can cause apical migration of the junctional epithelium, which in turn causes gingival recession, alveolar bone loss, and pocketing. The occlusion should be monitored at recare appointments to detect occlusal changes.
Possibly the most important evaluation tool to evaluate the health and success of the implant is dental radiographic images. They are the most reliable of all the conventional periodontal indices for evaluating failing implants. A mobile implant may display a narrow, radiolucent space surrounding the implant–bone interface. Radiographic images can assess bone height and density and show the functional relationship between the prosthesis, implant, and abutment components. It is suggested that radiographic images (excluding the baseline radiographic image taken 1 week post-surgery) be taken every 3 months after initial placement of the implant. After the first year, radiographic images should be taken once each year. In the last few years, cone-beam computed tomography (CBCT) has been used for measuring cortical bone thickness, as well as being utilized in post-operative imaging. However, recent studies acknowledge its limitations such as overestimating the vertical distance between the top of the implant and the crestal bone.
DEBRIDEMENT
In addition to regular self-care procedures, a periodic, professional oral prophylaxis is required to maintain a healthy oral environment. Professional dental prophylaxis is essential in every periodontal maintenance case. For dental implant plaque and calculus removal, only instruments that do not damage the implant surfaces may be used. In commercial use and form, pure titanium is soft, non-magnetic, and passive. These metallic surfaces develop a layer of titanium oxide that does not undergo any further breakdown under physiologic situations. Damage can lead to changes in the surface chemistry of the material, resulting in corrosion. Surface roughness and corrosion facilitate plaque retention, ultimately compromising the implant. It is therefore imperative that no oral health maintenance procedure directly affect this titanium oxide surface layer.
Conventional metal curettes, as well as sonic and ultrasonic scalers, cause considerable changes to the implant surface. Only instruments made of plastic, graphite, nylon, or those with a Teflon® coating should be in contact with the implant. The use of a dissimilar metal (such as stainless steel) on titanium may lead to corrosion. The use of these dissimilar metals on implant surfaces have been studied in vitro, comparing the number of human gingival fibroblasts attaching to the surface of a commercially pure titanium-alloy curette. Results showed a significant reduction in the number of fibroblasts attaching to titanium implants that had been scaled with the stainless-steel curette when compared to the plastic and titanium scalers.
Ultrasonic instrumentation continues to be contraindicated with dental implants. Ultrasonic scalers may severely disrupt the titanium dioxide surface, leading to a multitude of grooves and a roughened surface, which can lead to further plaque retention and a compromised implant. A study utilizing a modified ultrasonic instrument with a custom-designed delvin plastic tip showed that the standard ultrasonic instrument caused considerable scratching and gouging to the titanium implant. Shallow scratches made with the metal ultrasonic could be polished smooth, but the deeper scratches could not. The modified ultrasonic instrument produced noticeable but minimal changes that when polished did not appear to be microscopically different from the polished control. The modified ultrasonic instrument may be a promising device for maintenance of the dental implant. No definite answer can be made concerning ultrasonic use for implants at this time.
Although air polishing on implant surfaces was controversial in the past, recent studies have shown air polishing to be effective and safe for maintenance procedures.
After calculus deposits have been removed, the prosthesis and abutments may be selectively polished with a rubber cup and a nonabrasive fine polishing paste. Rubber cup polishing alone appears to be the least abrasive treatment using a prophylaxis paste, commercial implant pastes, or tin-oxide. However, paste deposits will be left on the implant surfaces. A rubber point may also be used. After polishing, the implant surfaces should be gently irrigated with water to avoid any adverse tissue healing. An antimicrobial solution should be applied to the peri-implant tissues.
If a dental implant is displaying increased probing depths, bleeding, or any other indication of the onset of failure, a controlled drug delivery system can be applied. These systems may contain a tetracycline-loaded fiber that is designed to slowly release the antibiotic over a 10-day period. The fibers can be used in single or multiple sites and may provide additional benefits to conventional scaling and root planing.
A strict prophylaxis recare schedule should be established and maintained to monitor the oral health findings in dental implant patients. The patient is often seen for comprehensive oral hygiene instructions and soft-tissue examination within the first week after the prosthesis is placed. A follow-up visit is scheduled for 1 month later. At this appointment, the clinician reviews the adequacy of self-care procedures and re-evaluates the health of the peri-implant tissues. After the 1-month follow-up, a 3-month recare schedule is suggested for a 1-year duration. Depending on patient self-care and the individual’s periodontal status, the patient may then be placed on a 6-month recare schedule after the first year. During the first 2 years, no more than 6 months should elapse between recare visits.
SUMMARY
The dental professional’s role is to determine the dental implant patient’s individual and specific self-care needs. Recommendations and instructions to patients are often determined by the prosthesis design, location, and angulation of the implants, the length and the position of the transmucosal abutments, and other factors such as patient habits (ie, smoking), motivation to perform consistent self-care, and the patient’s manual dexterity. It is important to recommend individualized auxiliary aids to gain and maintain appropriate self-care and compliance. To ensure optimal peri-implant health, the patient must maintain daily biofilm removal and regular professional care. To achieve long-term success, it is important to maintain prophylaxis recare schedule at which evaluations are performed to assess gingival, bone, and implant health.
ABOUT THE AUTHORS
Connie Myers Kracher, PhD, MSD
Associate Professor of Dental Education, Chair of the Department of Dental Education, and Director of the Dental Assisting Program at Indiana University - Purdue University, Fort Wayne, Indiana. She holds a PhD from Lynn University in Boca Raton, Florida, and a Master of Science in Dentistry from the Indiana University School of Dentistry in Oral Biology. Dr. Kracher is a frequent contributor to the Dental Assistant Journal and author of several ADAA courses: Sports Related Dental Injuries & Sports Dentistry, Oral Health Maintenance of Dental Implants, and Blood Pressure Guidelines and Screening Techniques.
Wendy Schmeling Smith, RDH, BSEd
Wendy Schmeling Smith, RDH, BSEd received her baccalaureate degree in education from Indiana University – Purdue University, Indianapolis. She is a licensed dental hygienist in private practice in Indianapolis, Indiana.
REFERENCES
American Academy of Periodontology. Maintenance and treatment of dental implants (position paper). J Periodontol. 1995;66:23-29.
American Academy of Periodontology. Dental implants in periodontal therapy (position paper). J Periodontol. 2000;71:1934-1942.
Bauman GR, Mills M, Rapley JW, Halmon WW. Implant maintenance: debridement and peri-implant home care. Compendium. 1991;12(9):644,646,648.
Balshi TJ. Hygiene maintenance procedures for patients treated with the tissue integrated prosthesis. Quintessence Int. 1986;17(2):95-102.
Biesbrock, Bartizek RD, Walters PA, et al. Clinical evaluations of plaque removal efficacy: an advanced rotating-oscillating power toothbrush versus a sonic toothbrush. J Clin Dent. 2007;18(4):106-111.
Babbush CA, Hahn JA, Krauser JT, Rosenlicht JL. Dental Implants. The Art and Science. 2nd ed. Saunders: Maryland Heights, MO; 2011.
Becker W, Becker BE, Newman MG, Nyman S. Clinical microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990;5(1):31-38.
Brånemark PI, Zarb GA, Albrektsson T. Tissue-Integrated Prosthesis Osseointegration in Clinical Dentistry. Chicago, IL: Quintessence Publishing; 1985:14-25.
Brough Muzzin KM, Johnson R, Carr P, Daffron P. The dental hygienist’s role in the maintenance of osseointegrated dental implants. J Dent Hyg. 1988;62(9):448-453.
Dmytryk J, Fox S, Moriarty J. The effects of scaling titanium implant surfaces with metal and plastic instruments on cell attachment. Abstract from Research Forum of the American Academy of Periodontology 75th Annual Meeting, Oct 26, 1989.
Fox S, Moriarty J, Kusy RP. The effects of scaling a titanium implant surface with metal and plastic instruments: an in vitro study. J Periodontol. 1990;61(8):485-490.
Friedman LA. Oral hygiene for dental implant patients. Tex Dent J. 1991;108(5):21-29.
James RA. Peri-implant considerations. Dent Clin North Am. 1980;24(3):415-420.
Kawashima H, Sato S, Kishida M, et al. Treatment of titanium dental implants with three piezoelectirc ultrasonic scalers: an in vivo study. J Periodontal. 2007;78(9):1689-1694.
Koumijian JH, Kerner J, Smith RA. Implants: hygiene maintenance of dental implants. Ill Dent J. 1991;60(1):54-59.
Kracher CM. Peri-implant postoperative treatment considerations to prevent peri-implantitis. World Dental Reporter. Spring 2011;3:17-19.
Kracher CM. 2012 Continuing Education: Current concepts in preventive dentistry. American Dental Assistants Association.
Kwan JY, Zablotsky MH, Meffert RM. Implant maintenance using a modified ultrasonic instrument. J Dent Hyg. 1990;64(9):422,424-425,430.
Meffert RM. The soft tissue interface in dental implantology. J Dent Educ. 1988;52(12):810-811.
Mioduski TE Jr, Guinn NJ. Dental implants. Permanent replacement for lost teeth. AORN J. 1990;51(3):729-734.
Misch CE. Contemporary Implant Dentistry. 3rd ed. St. Louis, MO: Mosby; 2008.
Nimmons KJ. The expanding esthetic practice: implant maintenance - part 2. Comp Esthetics Restor Prac. 2005; May:2-5.
Orton GS, Steele DL, Wolinsky LE. The dental professional’s role in monitoring and maintenance of tissue-integrated prostheses. Int J Oral Maxillofac Implants. 1989;4(4):305-310.
Papaspyridakos P, Chen CJ, Singh M, et al. Success criteria in implant dentistry: a systematic review. J Dent Res. 2012;91(3):242-248.
Rapley JW, Swann RH, Halmon WW, Mills MP. The surface characteristics produced by various oral hygiene instruments and materials on titanium implant abutments. Intl J Oral Maxillofac Implants. 1990;5(1):47-52.
Ramaglia L, di Lauro AE, Morgese F, Squillace A. Profilometric and standard error of the mean analysis of rough implant surfaces treated with different instrumentations. Implant Dent. 2006;15(1):77-82.
Rasmussen RA. The Branemark System of Oral Reconstruction. A Color Atlas. St. Louis, MO: Ishiyaku EuroAmerica, Inc; 1992.
Razavi T, Palmer RM, Davies J, et al. Accuracy of measuring the cortical bone thickness adjacent to dental implants using cone beam computer tomography. Clin Oral Implants Res. 2010;21(7):718-725.
Renvert S, Lesse J, Dahlen G, et al. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient perio-implant infections: a randomized clinical trial. J Clin Periodontol. 2006;33(5):362-369.
Sato S, Kishida M, Ito K. The comparative effect of ultrasonic scalers on titanium surfaces: an in vitro study. J Periodontol. 2004;75(9):1269-1273.
Taylor TD. Dental Implants: Are They for Me? 2nd ed. Quintessence Books: 1993.
VanOrden AC. Corrosive Response of the Interface Tissue to 316L Stainless Steel, Titanium-Based Alloys, and Cobalt-Based Alloys. The Dental Implant. PSG Publishing Co., Inc. 1985:1-24.
Vehemente VA, Chuang SK, Daher S, et al. Risk factors affecting dental implant survival. J Oral Implantol. 2002;28(2):74-81.
Van Steenberghe D. Periodontal aspects of osseointegrated oral implants and modum Brånemark. Dent Clin North Am. 1988;32(2):355-370.
Wilkins EM, Wyche C. Clinical Practice of the Dental Hygienist. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.