You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Various factors have propagated the successful use of zirconia in dentistry in the past decade. These include the rising cost of precious metals, an increased demand for esthetics, advancements in digital design and laboratory milling, new bonding techniques, and developments in dental zirconia. One of the pioneering articles that described the type of stabilized zirconia used in dentistry was titled “Ceramic Steel?” published in Nature in 1975.1 Zirconia was compared to steel because of the toughness it achieves through a process termed transformation toughening.
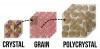
Zirconia is a ceramic, which is defined as a material composed of a metal (ie, zirconium) and a nonmetal (ie, oxygen). Generally, the metal and nonmetal atoms in a ceramic can be arranged in amorphous, non-ordered glass structures (ie, porcelain) or in ordered crystalline structures (ie, zirconia) (Figure 1). As per basic chemistry, atoms in a crystal may be arranged in different crystalline structures. To demonstrate this point, the author (NL) recalls when his high-school chemistry teacher once organized several layers of tennis balls into a large plastic bin. After creating an initial layer of tennis balls at the base of the bin, he carefully placed new tennis balls into the depressions made where four balls intersected in the layer below. He then removed all the tennis balls other than those in the bottom layer and refilled the bin, this time placing each tennis ball directly on top of the ball below it. In this example, the tennis balls represent atoms, and these two arrangements of balls represent different crystalline structures, with the first arrangement being more compact than the second (Figure 2). Similarly, zirconia may be arranged in different crystalline structures, known as tetragonal and monoclinic phases, with the first more compact than the second. When observing zirconia under a microscope, individual units will be seen (Figure 3). These units are called grains and represent a volume of material composed of a single crystalline structure oriented in a particular direction. In other words, if hundreds of bins were filled with tennis balls and these bins were then randomly dropped into a swimming pool, each bin would represent a grain (Figure 3).
When clinicians receive a zirconia crown from a laboratory, it has been manipulated so each grain is in the tetragonal phase. The unique ability of zirconia is that forces (such as biting, bur grinding, particle abrasion, etc) will cause tetragonal grains to transform into larger monoclinic grains. Thus, if a crack is started in zirconia, the local expansion of tetragonal grains into monoclinic grains will compress the crack, halting its progression (Figure 4). This phenomenon is called transformation toughening, and it accounts for the higher fracture toughness of zirconia (4.9 MPa m1/2) compared to other dental ceramics such as lithium disilicate (2.8 MPa m1/2).2
Degradation Over Time
As noted, transformation toughening strengthens zirconia into a durable restorative material. Unfortunately, the same mechanism that toughens zirconia also may lead to its long-term degradation. Aside from external forces causing phase transformation, zirconia in an aqueous solution can also transform spontaneously over time in a process called low temperature degradation (LTD). In LTD, the transformation begins at the surface of the zirconia as tetragonal grains spontaneously transform to monoclinic grains. Because of the increase in grain size following transformation, surrounding grains will push against each other causing cracking between grains and surface roughening.3 In a crown, the cracking may weaken the zirconia, and the increased roughness could cause wear of the opposing tooth. Therefore, it is important to know what changes occur in zirconia as it ages and whether these changes will lead to failure of zirconia restorations.
Although it has been proven that LTD occurs in laboratory studies and explanted hip implants, the extent of transformation and the clinical repercussions in dental zirconia are mostly theoretical. In one recent study, zirconia specimens were stored in water 1,500 days and it was determined that transformation occurred at a rate of ~0.5 µm per year.4 Other studies have simulated accelerated LTD by placing zirconia in an autoclave. A recent study reported a 30% decrease in crown fracture strength following 100 hours of accelerated aging.5 The reduced strength value is still in excess of the clinically successful material lithium disilicate. Slight increases in roughness have been measured against artificially aged zirconia; however, simulated wear testing showed no difference in opposing enamel wear against aged and non-aged zirconia.6 In summary, although LTD occurs in zirconia, there is insufficient evidence to demonstrate that significant transformation occurs in the mouth. Furthermore, simulated aging suggests that the property changes may not have clinical significance.
Weakening Effects of Particle Abrasion and Diamond Bur Adjustment
After a zirconia restoration is milled in the laboratory, it is sintered in a furnace to achieve its final strength. This firing causes all of the grains to be arranged in the tetragonal phase. When a clinician receives a zirconia crown in the office, some adjustments will likely be needed, such as alumina particle abrasion to prepare the crown for bonding or bur grinding the crown to adjust the occlusion or proximal contacts of the crown. Particle abrasion or bur grinding will introduce microscopic cracks in the zirconia as well as transform some of the surface tetragonal zirconia into monoclinic zirconia.7 A recent study showed that the mechanical damage that occurs when adjusting zirconia with a coarse diamond bur (Figure 5) reduces the strength of zirconia.8 Polishing the restoration following adjustment may remove some of the surface scratching and help regain the strength of the zirconia. Particle abrasion, however, does not have an immediate effect on the strength of the zirconia and, in some cases, may actually strengthen the zirconia.7,9
It is likely that transformation of zirconia counteracts the microcracks introduced from abrasion or grinding, minimizing the damage to the zirconia surface. Some manufacturers and dental laboratories recommend “healing” the abraded or ground zirconia by heating it in a furnace to return all grains to the tetragonal phase. However, a recent study reported decreased strength of “healed” zirconia, because reheating ground or adjusted zirconia releases the compression on surface microcracks when the zirconia is transformed back to the tetragonal phase.9
Wear on Opposing Enamel
Clinical experience has produced cases of severe wear of enamel opposing porcelain-fused-to-metal (PFM) crowns (Figure 6). Because zirconia is significantly harder than veneering porcelain, predictions of enamel wear opposing full-contour zirconia were in excess of porcelain. Surprisingly, laboratory studies of simulated enamel wear against zirconia showed significantly lower values than enamel opposing veneering porcelain.10,11 Microscopic evaluation of the zirconia and porcelain revealed that throughout the wear process, the surface of the porcelain fractured and roughened, whereas the zirconia surface remained sound and smooth. The roughening of the porcelain surface caused abrasive wear of the opposing enamel. Therefore, if zirconia is more wear-friendly than veneering porcelain, full-contour zirconia crowns should be no more damaging than time-tested PFM crowns.
Further evaluation revealed that glaze layers placed on zirconia would also roughen and wear opposing enamel.10 Evaluation of zirconia adjusted with a diamond finishing bur produced slightly greater opposing enamel wear than polished zirconia; however, the amount of wear may not actually be clinically relevant.11 Evaluation of the zirconia itself revealed that it did not experience material wear.10,11 This observation caused concern among gnathologists, who speculated that contacts between zirconia crowns and natural teeth would wear at a slower rate than natural teeth, ultimately affecting the patient’s occlusion. In vivo studies of zirconia-enamel wear, however, have shown that zirconia-enamel contacts, in fact, wear slightly more than natural enamel-enamel contacts.12
Chipping of Porcelain Veneered to Zirconia Core
A major clinical concern of porcelain-fused-to-zirconia (PFZ) crowns and fixed partial dentures is fracturing of veneering porcelain. A 2010 study reported a higher incidence of veneer chipping in PFZ restorations (54%) than in PFM restorations (34%).13 Several explanations have been offered for the increased prevalence of chipping, as well as methods to avoid these modes of failure: (1) Because zirconia has a lower coefficient of thermal expansion than dental alloys, placing the veneering porcelain used for PFM crowns on PFZ crowns would not place the veneering porcelain in a proper state of pre-compression.14 As a result, zirconia-specific veneering porcelain should be used. (2) PFZ crowns designed with non-anatomic copings (ie, copings that follow the shape of the tooth preparation rather than the final contours of the crown) do not provide adequate support of the veneering porcelain.15 Therefore, all zirconia copings should be designed with an anatomic coping. (3) Zirconia is an insulator. When a PFZ is removed from the furnace after application of the veneering porcelain, the porcelain exposed to the environment releases heat easily whereas the porcelain in contact with zirconia traps heat. If the PFZ crown is removed from the furnace immediately, there is a gradient of heat between the rapidly cooling external surface of the porcelain and the internally insulated surface of the porcelain. As a result, thermal waves travel through the porcelain, which creates corresponding compression-tension waves. When the compression-tension waves encounter defects in the porcelain, internal microcracks may be formed that predispose the veneering porcelain to fracture.16 To prevent the compression-tension waves, PFZ crowns should be slow-cooled when removed from the furnace.
Difficult Removal of Zirconia Crowns
Removing a full-contour zirconia crown can be one of the most frustrating clinical experiences with dental zirconia. Several manufacturers have developed burs specifically designed to remove zirconia crowns that have been shown to be more effective than normal diamond burs.17 An important consideration when achieving an endodontic access through a zirconia crown is that coarse burs tend to cause edge chipping around the cutting area, which may predispose the crown to eventual fracture (Figure 7).17 Therefore, a fine diamond bur should be used when accessing through a zirconia crown for root canal treatment.
Opacity Not Ideal for Esthetic Restorations
Ceramics containing glass are translucent due to their lower atomic density (Figure 1). Because zirconia is purely crystalline without glass content, it is opaque. The opacity of zirconia is acceptable for many restorations, particularly those in posterior areas of the mouth. The esthetic demands of some patients, however, require the use of a restorative material that can match the natural translucency of tooth structure.
One method of increasing zirconia translucency is to reduce its grain size via a shorter sintering time.18 The trade-off, however, is that if the grain size is too small, the zirconia will be unable to perform transformation toughening and be less resistant to fracture. Recently, translucent zirconia has been fabricated with increased yttria content, which has stabilized the cubic phase.19 Translucent zirconia materials have reported strength values less than those of typical zirconia; however, their flexural strength is still higher than lithium-disilicate materials.20 Many of their other clinical properties are untested.
Bonding to Zirconia
Because zirconia does not contain glass it cannot be etched with hydrofluoric acid, so micromechanical retention may be obtained through aluminum oxide (Al2O3) particle abrasion (Figure 8). Also, silane will not bond to zirconia (unless it is coated with silica). Therefore, the molecule 10-methacryloyloxydecyl dihydrogen phosphate (MDP) is used to bond zirconia to resin cements. The combination of Al2O3 particle abrasion and MDP application has been shown to work as an effective bonding protocol for zirconia.21
Conservative Tooth Preparation for Monolithic Zirconia Restorations
The high strength of zirconia enables tooth preparation to be much more conservative than with lithium-disilicate restorations. Some manufacturers have suggested a minimum reduction of 0.5 mm for monolithic zirconia. A recent study demonstrated that monolithic zirconia crowns with 0.8 mm occlusal reduction demonstrated lower crown fracture strength than PFM crowns with 1.5 mm reduction. One-millimeter monolithic zirconia crowns showed a statistically equivalent crown fracture strength to the 1.5-mm reduced PFM crowns.22 Margins for monolithic zirconia restorations are typically prepared as a chamfer margin; however, a laboratory study has shown higher crown fracture strength using a knife-edge margin than a chamfer margin.23 Practically, it is difficult to produce a true knife-edge margin when milling zirconia because of chipping of the thin edges during fabrication.
Conclusion
Clinical conclusions can be summarized as follows:
• There is insufficient evidence to suggest that low-temperature degradation will affect the lifetime survival (>15 years) of zirconia restorations.
• Particle abrasion does not have an immediate effect on the strength of zirconia.
• Diamond adjustment of zirconia can reduce its strength; however, heat healing is not effective at returning its original strength.
• Zirconia is more wear-friendly to opposing enamel than veneering porcelain; however, enamel-zirconia wear is slightly higher than enamel-enamel wear.
• In porcelain-veneered-to-zirconia crowns, an anatomic coping design should be used and zirconia-specific porcelain should be fired onto the zirconia and slow-cooled.
• Zirconia crowns are most effectively removed with zirconia-specific burs, and fine diamond should be used to access through zirconia crowns.
• New formulations of zirconia crowns promise the translucency to achieve esthetic restorations.
• Bonding to zirconia is possible with MDP.
• Zirconia crowns should be prepared with 1-mm occlusal reduction, and a knife-edge margin may be acceptable based on current evidence.
ABOUT THE AUTHORS
Nathaniel Lawson, DMD, PhD
Assistant Professor and Director of Biomaterials Residency, University of Alabama Birmingham (UAB) School of Dentistry, Birmingham, Alabama
Chin Chuan Fu, DDS, MS
Assistant Professor, University of Alabama Birmingham (UAB) School of Dentistry, Birmingham, Alabama
REFERENCES
1. Garvie RC, Hannink RH, Pasco RT. Ceramic steel? Nature. 1975;258(5537):703-704.
2. Quinn JB, Sundar V, Lloyd IK. Influence of microstructure and chemistry on the fracture toughness of dental ceramics. Dent Mater. 2003;19(7):603-611.
3. Lawson S. Environmental degradation of zirconia ceramics. J Euro Ceram Soc. 1995;15(6):485-502.
4. Keuper M, Berthold C, Nickel KG. Long-time aging in 3 mol.% yttria-stabilized tetragonal zirconia polycrystals at human body temperature. Acta Biomater. 2014;10(2):951-959.
5. Nakamura K, Harada A, Kanno T, et al. The influence of low-temperature degradation and cyclic loading on the fracture resistance of monolithic zirconia molar crowns. J Mech Behav Biomed Mater. 2015;47:49-56.
6. Burgess JO, Janyavula S, Lawson NC, et al. Enamel wear opposing polished and aged zirconia. Oper Dent. 2014;39(2):189-194.
7. Ozcan M, Melo RM, Souza RO, et al. Effect of air-particle abrasion protocols on the biaxial flexural strength, surface characteristics and phase transformation of zirconia after cyclic loading. J Mech Behav Biomed Mater. 2013;20:19-28.
8. Pereira G, Amaral M, Cesar PF, et al. Effect of low-temperature aging on the mechanical behavior of ground Y-TZP. J Mech Behav Biomed Mater. 2015;45:183-192.
9. Passos SP, Linke B, Major PW, Nychka JA. The effect of air-abrasion and heat treatment on the fracture behavior of Y-TZP. Dent Mater. 2015;31(9):1011-1021.
10. Janyavula S, Lawson N, Cakir D, et al. The wear of polished and glazed zirconia against enamel. J Prosthet Dent. 2013;109(1):22-29.
11. Lawson NC, Janyavula S, Syklawer S, et al. Wear of enamel opposing zirconia and lithium disilicate after adjustment, polishing and glazing. J Dent. 2014;42(12):1586-1591.
12. Mundhe K, Jain V, Pruthi G, Shah N. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J Prosthet Dent. 2015;114(3):358-363.
13. Heintze SD, Rousson V. Survival of zirconia- and metal-supported fixed dental prostheses: a systematic review. Int J Prosthodont. 2010;23(6):493-502.
14. Aboushelib MN, de Jager N, Kleverlaan CJ, Feilzer AJ. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dent Mater. 2005;21(10):984-991.
15. Altamimi AM, Tripodakis AP, Eliades G, Hirayama H. Comparison of fracture resistance and fracture characterization of bilayered zirconia/fluorapatite and monolithic lithium disilicate all ceramic crowns. Int J Esthet Dent. 2014;9(1):98-110.
16. Benetti P, Kelly JR, Sanchez M, Della Bona A. Influence of thermal gradients on stress state of veneered restorations. Dent Mater. 2014;30(5):554-563.
17. Lin C, Cakir C, Burgess JO, et al. Comparison of cutting effectiveness of diamond burs with Zr blocks. J Dent Res. 2014;93(spec iss A): Abstract 707.
18. Kim MJ, Ahn JS, Kim JH, et al. Effects of the sintering conditions of dental zirconia ceramics on the grain size and translucency. J Adv Prosthodont. 2013;5(2):161-166.
19. Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014;30(10):1195-1203.
20. BruxZir® Anterior Solid Zirconia. Glidewell Laboratories website. http://www.glidewelldental.com/dentist/services/all-ceramics-bruxzir-anterior.aspx. Accessed May 19, 2016.
21. Yang B, Barloi A, Kern M. Influence of air-abrasion on zirconia ceramic bonding using an adhesive composite resin. Dent Mater. 2010;26(1):44-50.
22. Sun T, Zhou S, Lai R, et al. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J Mech Behav Biomed Mater. 2014;35:93-101.
23. Beuer F, Aggstaller H, Edelhoff D, Gernet W. Effect of preparation design on the fracture resistance of zirconia crown copings. Dent Mater J. 2008;27(3):362-367.