You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Hypothermia during anesthesia is the most common perioperative thermal disturbance.1 It is not uncommon for patients to become cold and exhibit uncontrollable episodes of shaking and shivering. These events are both troubling and perplexing to the anesthesia provider. The purposes of this article are to review the processes of heat loss and thermoregulation and to use this information to properly care for patients during sedation and general anesthesia. Although malignant hyperthermia is a relatively rare occurrence, we will also summarize current information on its pathogenesis and management.
PHYSICS OF HEAT TRANSFER
Heat loss occurs primarily from the skin of a patient to the environment through several processes, including radiation, conduction and convection, and evaporation.2 Of these, radiation is most significant and accounts for ~60% of total heat loss. Radiation is emitted in the form of infrared rays, a type of electromagnetic wave. Heat from core body tissues is transported in blood to subcutaneous vessels, where heat is lost to the environment through radiation. This manner of heat loss is the basis for the familiar technology used to sense and identify the locations of persons in buildings who are out of normal view. Radiation is the major source of heat loss in most surgical patients.
Conduction refers to loss of kinetic energy from molecular motion in skin tissues to surrounding air. Water absorbs far more conducted heat than air, and this accounts for more rapid hypothermia during accidental drowning, as well as the efficacy of water baths to cool hyperthermic patients. For this to be effective, warmed air or water must be moved away from the skin surface by currents, a process called convection. This accounts for the cooling effect of wind and laminar air flow in many surgical suites. Conduction and convection account for ~15% of body heat loss.
Roughly 22% of heat loss occurs by evaporation, as energy in the form of heat is consumed during the vaporization of water. Water evaporates from the body even when not sweating, but mechanisms that enhance sweating increase evaporation. As long as skin temperature is greater than its surroundings, radiation and conduction provide heat loss. At very high environmental temperatures, these processes cannot work, and evaporation is the only manner in which heat can be dissipated. This generally is not the case in the clinical setting.
FUNDAMENTAL PROCESSES IN THERMOREGULATION
Skin temperature rises and falls with the temperature of a patient’s surroundings. However, the temperature of deep body tissues, that is, the core temperature, remains relatively constant at 98.0°F to 98.6°F (37°C). In fact, core temperature normally remains between 97°F and 100°F, even while environmental temperatures fluctuate from as low as 55°F to as high as 130°F.2 This is due to a remarkable thermoregulatory system that is conventionally organized into three components: afferent sensing, central control, and efferent responses.
Afferent Sensing
Afferent input is triggered by thermal-sensitive cells (receptors) found not only in skin but throughout most of the body. Receptors for cold are anatomically and physiologically distinct from those for heat. Cold receptors are excited by temperatures below a set threshold and generate impulses that travel mainly via Ad Aδ (A-delta) nerve fibers. Temperatures above threshold excite heat receptors that generate impulses along unmyelinated C fibers, which also conduct pain sensation.3 For this reason, patients frequently are unable to discriminate between sharp pain and intense heat. Information is then integrated at several levels within the spinal cord and brain, finally arriving at the primary thermoregulatory center within the hypothalamus.
Central Control
Although some integration and temperature regulation may occur at the spinal cord level, the hypothalamus is the primary center for thermoregulatory control, integrating most afferent input and coordinating the various efferent outputs required to maintain a normothermic level. The precise manner by which the body establishes temperature thresholds is unclear, but it appears to involve the interactions of several neurotransmitters, including norepinephrine, dopamine, 5-hydroxytryptamine (serotonin), acetylcholine, prostaglandin E1, and other neuropeptides. Additional factors such as circadian rhythm, exercise, food intake, infection, thyroid dysfunction, menstrual cycle, anesthetics, and and other drugs are known to alter temperature thresholds.1-3
Efferent Responses
Behavior is the most effective response for thermoregulation. This includes dressing appropriately, modifying environmental temperature, assuming bodily positions that diminish or enhance heat loss, and increasing voluntary movement to generate heat production. Obviously, these considerations must be addressed before a patient is anesthetized and will be considered later in this review.
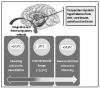
As temperature receptors transmit information to the hypothalamus, it is integrated and compared with threshold settings. Values above or below these thresholds determine the efferent response that is generated (Figure 1). Efferent outputs from the hypothalamus regulate body temperature by altering subcutaneous blood flow, sweating, skeletal muscle tone, and overall metabolic activity. Heat loss is promoted by vasodilation and sweating, while heat is conserved by inhibiting these processes. Production of heat (thermogenesis) is promoted by shivering and increases the overall metabolic rate. These influences are further explained and summarized in Table 1.
DRUG INFLUENCES ON THERMOREGULATION
Causes for inadvertent hypothermia include not only patients’ exposure to a cold room environment and their inability to initiate behavior responses, but the proclivity of anesthetics to promote heat loss. Volatile anesthetics, propofol, and older opioids such as morphine and meperidine promote heat loss through vasodilation. This process is compounded further by the fact that these drugs, as well as fentanyl and its derivatives, directly impair hypothalamic thermoregulation in a dose-dependent manner. Opioids also depress overall sympathetic outflow, which further inhibits any attempts at thermoregulation. The depressant effect on the hypothalamus results in an elevated threshold for heat response, along with a diminished threshold for cold response such as vasoconstriction and shivering. Therefore, opioids widen the normal interthreshold range from ~0.2°C to as much as 4°C, and patients are unable to adjust to cold environments and heat loss resulting from vasodilation (Figure 1). It is notable that nitrous oxide depresses thermoregulation to a lesser extent than equipotent concentrations of the volatiles, and midazolam has minimal or no influence.3 Presumably this would be true for other benzodiazepines as well.
Following induction of general anesthesia, the decline in body temperature occurs in three phases. The greatest decline occurs during the first half hour or phase 1. Normally body heat is maintained in an unevenly distributed manner; the temperature of core tissues is 2°C to 4°C greater than skin temperature. Following anesthesia induction, however, vasodilation combined with a lowered cold threshold in the hypothalamus allows a redistribution of body heat from core tissues to skin, where heat is lost primarily through radiation. Phase 2 commences after approximately 1 hour, as core temperature decreases at a slower rate and proceeds in a linear manner as heat lost from the body exceeds heat production. Finally, after 3 to 5 hours, phase 3 commences, as an equilibrium is reached where heat loss is matched by heat production and thermoregulated vasoconstriction commences to function.1,3
Curiously, regional anesthesia also produces hypothermia. In dentistry, the region of blockade is so small that it presents no concern. Regional anesthesia in medicine however produces similar patterns of heat loss and hypothermia as are produced by general anesthesia. Hypothermia is very common following spinal and epidural anesthesia. Blockade of afferent fibers from large regions obviously prevents cold input to the hypothalamus. However, despite the fact that locally injected anesthetics have no direct action on the hypothalamus, the thermoregulatory center nevertheless becomes impaired. (This does not occur following the intravenous administration of lidocaine for cardiac arrhythmias.) For reasons yet to be explained, the thermoregulatory center incorrectly judges skin temperature in blocked regions to be abnormally elevated.1 The net result is that the interthreshold range increases 3 to 4 times (from 0.2°C to 0.6°C to 0.8°C), which of course is significantly less than the 20-fold increase that can be produced by general anesthesia (from 0.2°C to 4°C) (Figure 1). Despite this drop in core temperature, patients generally feel warm because the hypothalamus misinterprets skin temperature. In fact, patients may become hypothermic enough to commence shivering despite their subjective feeling of warmth. The concomitant use of sedation not only compounds the depression of thermoregulation but obtunds the patient’s subjective sensations.
CONSEQUENCES AND BENEFITS OF HYPOTHERMIA
Hypothermia is defined as a core temperature <35°C and may be classified according to severity based on temperatures below this reading.4 Perioperative hypothermia may produce a multitude of deleterious effects, which are summarized in Table 2.5
Other than shivering, the most common complications associated with hypothermia are (1) a threefold increase in morbid myocardial events,6 (2) a threefold increase in the risk of surgical wound infection,7 and (3) an increase in blood loss and transfusion requirements.8 Adverse cardiovascular events can follow intraoperative depression of cardiac output and heart rate, as well as a rebound during the postoperative period. Hypothermia during the postoperative period markedly impairs thermal comfort, and physiologic stress leads to increases in heart rate, blood pressure, and oxygen consumption.9 Hypothermia most likely contributes to wound infection through impairment of immune function and through thermoregulatory vasoconstriction, which, in turn, diminishes oxygen delivery to surgical sites.3 Fever normally increases leukocyte mobilization, but this protective response is lost during hypothermia. Even mild hypothermia hampers blood clotting. The most significant factor is a cold-induced inhibition of platelet function, but the activities of enzymes that drive the coagulation cascade are also impaired.10
Drug metabolism can be markedly decreased by hypothermia. During a constant infusion of propofol, plasma concentration is increased by 30% in patients who are 3°C hypothermic.11 The pharmacodynamics and pharmacokinetics of muscle relaxants and volatile anesthetics are likewise altered. Minimum alveolar concentration (MAC) is reduced by 5% for each °C below normal.3
Although hypothermia is generally regarded as deleterious, it can be beneficial in some situations. Hypothermia decreases the overall metabolic rate by 8% per °C to about half the normal rate at 28°C.3 Oxygen demand drops and those tissues that have high oxygen consumption normally, such as brain and heart, have a proportionally greater reduction of oxygen use. This allows aerobic metabolism to continue through greater periods of compromised oxygen supply, thereby reducing the production of anaerobic byproducts such as superoxide radicals and lactate. Additional protection can be attributed to decreased release of excitatory neurotransmitters, reduced synthesis and release of kinases and proinflammatory cytokines, and decreased apoptosis.12 In addition, hypothermia lowers intracranial pressures and cerebral perfusion pressure.3
Substantial protection against cerebral ischemia and hypoxia can be gained by providing a 1°C to 3°C reduction in core temperature. Therapeutic hypothermia is used in many neurosurgery cases and in other procedures such as coronary artery bypass surgery in which tissue ischemia can be anticipated. Therapeutic hypothermia has also been shown to improve outcome during recovery from cardiac arrest and recovery.13,14
MALIGNANT HYPERTHERMIA
Although regulation of temperature and the consequences of hypothermia are the primary focus of this review, a brief discussion of malignant hyperthermia is worthwhile. Malignant hyperthermia (MH) is a potentially life-threatening event triggered by the administration of halogenated anesthetics and depolarizing neuromuscular blocking agents. It is significant that nitrous oxide, local anesthetics, intravenous anesthetics, and competitive neuromuscular blocking agents have not been implicated. Susceptibility is primarily attributed to an autosomal dominant gene that encodes an abnormal ryanodine receptor (RYR-1) in skeletal muscle. These receptors consist of a complex of calcium channels and sarcoplasmic reticulum that regulate the release of calcium ions from storage sites. The initiating event in malignant hyperthermia is an uncontrolled release of calcium ions, leading to accelerated muscle metabolism and subsequent clinical features that include contracture, rigidity, severe hyperthermia, metabolic acidosis, and tachycardia. In addition to the genetic susceptibility mentioned previously, certain myopathies, including Duchenne Muscular Dystrophy, central core disease, neuroleptic malignant syndrome, and King-Denborough syndrome, present a risk for MH.15
The most widely used test for determining MH susceptibility is the halothane-caffeine contracture test (CHCT). This test is performed on biopsied skeletal muscle tissue, which is exposed to the anesthetic halothane and the drug caffeine. Testing is performed only in limited centers in the United States, but once an individual experiences a syndrome resembling malignant hyperthermia, testing should be performed. Relatives should also receive testing and counseling. Complete information regarding malignant hyperthermia, including details of the various tests, can be found at the website for the Malignant Hyperthermia Association (www.mhaus.org).
The initial clinical presentation of malignant hyperthermia includes muscle rigidity and unexplained elevations in end tidal carbon dioxide (EtCO2) and heart rate followed by increasing temperature. An elevated temperature alone seldom indicates MH. Trismus and masseter muscle spasm may be the initial event following succinylcholine administration.15 Treatment includes rapid cooling, 100% oxygen, and control of metabolic acidosis. However, the decline in fatality due to MH is largely attributable to the rapid intravenous administration of dantrolene (Dantrium H). This drug acts by inhibiting release of calcium ions from sarcoplasmic reticulum. (It is also available in oral formulations for the management of spasticity.) Intravenous dantrolene is formulated as 20 mg in a 70-mL vial, to which 60 mL sterile water is added. It is administered by IV push in increments of 1 to 2 mg/kg to 10 mg/kg total until symptoms subside. At ~$80 per vial and a shelf life of ~3 years, the cost to maintain an adequate number of vials is considerable and is likely indicated only for offices that provide general anesthesia by using volatile anesthetics or the planned administration of succinylcholine. For patients who are documented as susceptible, the use of local anesthesia and sedation regimens using nitrous oxide, benzodiazepines, propofol, and opioids does not present a risk for triggering an event.15 If volatile anesthetics are normally used in the office, the anesthesia machine should be aerated by removing vaporizers and delivering an oxygen flow of 10 L/min for about 5 minutes. New circuitry and carbon dioxide-absorbent canisters should be used. Malignant hyperthermia is more difficult to trigger and is less severe when it occurs in patients who are rendered mildly hypothermic. For patients susceptible to malignant hyperthermia, active warming should be avoided, and they should be allowed to become slightly hypothermic during surgery.3
PREVENTION AND MANAGEMENT OF HYPOTHERMIA
Hypothermia may cause significant discomfort in the awake patient. Recovery is prolonged not only because a sense of coldness alters mentation and delays awakening, but because drug metabolism is reduced. These considerations are compounded by the negative physiological influences addressed previously.
As with most complications in anesthesia, prevention is the best management. When volatile anesthetics and large amounts of intravenous agents other than benzodiazepines are used, it is not possible to totally prevent a drop in core temperature.
Temperature Monitoring
Temperature monitoring is a standard for patients undergoing general anesthesia, although very brief procedures (eg, <15 minutes) may be an exception.5 It is rarely indicated for moderate sedation but should be considered during deep sedation, especially for patients at risk for hypothermia such as small children, the elderly, and others who are noticeably frail. Disposable thermocouple and thermistor probes are available for monitoring core temperature. Preferred sites include tympanic membrane, esophagus, nasopharynx, and rectum. These sites constitute anatomical areas of highly perfused tissues, the temperature of which is uniform and high in comparison with the rest of the body.3,5 Skin surface monitoring is not considered accurate or reliable because greater fluctuations occur and it is less reflective of core temperature.
Prewarming
Strategies for reducing heat loss are predicated on the fact that ~90% of heat loss occurs through the skin. Inform your patient to dress appropriately on the day of surgery. Patients who are undergoing dental and/or oral maxillofacial procedures can arrive with warm, appropriate clothing covering most of their body. Discourage the use of shorts and shirts that do not cover most of the torso and upper extremities, and request the use of socks. The patient can be prewarmed before induction with forced-air systems to minimize the drop in core temperature that results from redistribution. With prewarming of the extremities, which are normally 2°C to 4°C lower than core temperature, patients will require less heat to warm them when the core-to-peripheral redistribution of heat occurs. It is an inexpensive way to reduce perioperative hypothermia by banking heat in otherwise cooler body areas. Warm cotton blankets do not increase peripheral body temperature significantly, but they will comfort the patient and will at least minimize normal heat loss by minimizing the patient’s skin exposure.
Room Conditions
The operating room temperature is the most important factor in influencing heat loss due to radiation and convection from skin, and to evaporation from surgical wounds when they are large. The operating room should be warmed to greater than 24°C (ie, 76°F) during induction and while the patient is prepped and draped. All patients become hypothermic if the room is below 21°C (ie, 70°F). Once warming devices have been applied to the patient, the room temperature can be lowered to comfortable levels for the staff. Forced-air systems placed over patients are most effective and provide both insulation and active cutaneous warming. Warming of patients via skin surface apposition is most effective intraoperatively, because it is during this time that anesthetic-induced vasodilation occurs, allowing heat to flow peripherally down a temperature gradient. Forced-air systems have been shown to preserve body heat and maintain normothermia, even during the longest and most invasive surgical procedures. Patient positioning is important in heat conservation. The more radially positioned the patient’s extremities, the greater the heat loss. Placing of the arms and legs medially and tucking the patients with blankets to maintain the extremities against the body will also diminish the amount of heat loss.
Warming of IV Fluids
Warming of fluids can only help to minimize heat loss. Unfortunately, it is not possible to warm patients by administering heated fluids because they cannot be administered at temperatures above normal body temperatures because of the potential for denaturing proteins. Warm fluids are probably of benefit only when large amounts are administered for fluid replacement. A liter of fluid at room temperature will reduce the mean body temperature by approximately 0.25°C.3 Warming of fluids can be accomplished by using fluid warmers attached to the intravenous tubing or with the use of warming cabinets.
POSTOPERATIVE CONSIDERATIONS
During the postoperative recovery period, the body’s thermal heat transfer situation is significantly different. As the anesthetic-induced peripheral vasodilation dissipates, thermoregulatory vasoconstriction commences. Heat transfer from the periphery to the central core tissues is significantly impaired by this vasoconstriction. Because postoperative thermoregulatory vasoconstriction decreases peripheral-to-core heat transfer, applied warming to the skin is not as effective as during surgery when patients are vasodilated. Likewise, you are less likely to warm a patient by using conductive methods that were useful during surgery. Hence, it is easier to maintain intraoperative normothermia than to rewarm patients postoperatively. Patients managed with regional anesthesia and sedation warm faster than those recovering from general anesthesia.
Shivering: Causes and Management
Postoperative shivering is a common complication following general anesthesia and also occurs intraoperatively during moderate and deep sedation. Even a small decrease of 0.5°C may induce shivering. Patients often identify feeling cold as one of the most unpleasant aspects of their treatment, sometimes worse than any pain associated with the procedure. Shivering is not only subjectively unpleasant but is physiologically stressful because it elevates blood pressure, heart rate, oxygen consumption, and plasma catecholamine concentrations. Moreover, shivering may aggravate pain and hinder wound closure by simply stretching surgical incisions.
The mechanism for thermogenic shivering is related to increased neuronal efferent outflow to skeletal muscle and subsequent feedback oscillations due to muscle spindle stretchreflexes.2 However, tremors experienced by patients may not always represent normal thermogenic shivering. Many additional causes of tremor have been attributed to uninhibited spinal reflexes, decreased sympathetic activity, pyrogen release, adrenal suppression, and pain. Tremor due to thermogenic shivering is generally tonic (continuous), whereas a clonic pattern (alternating contractions and relaxations) of tremor suggests other causes.3 Clonic tremors are associated with recovery from volatile anesthetics. The precise cause of this tremor pattern is unclear, but it may result from anesthetic-induced disinhibition of normal descending control over spinal reflexes.16 It is significant that pain may be a key factor in triggering tremor in patients who are otherwise normothermic.17 This is an important consideration during outpatient dental procedures. Likewise, extrapyramidal tremors due to drugs that block dopamine receptors in the basal ganglia should be considered. These include prochlorperazine, promethazine, and droperidol.
Postoperative shivering should be treated with warming of the patient, most effectively via forced-air systems. Warm blankets may not warm the patient significantly, as explained earlier, but they certainly make the patient subjectively feel better. Skin surface warmers are generally comforting to the patient, but the skin surface contributes only 20% to control shivering, and these adjuncts will increase skin temperature by only a few degrees centigrade. This will compensate for only a small amount of core hypothermia and usually will not prove effective in most patients with core temperatures much below 35°C.3
Postanesthetic shivering can also be treated with a variety of drugs, including clonidine, physostigmine, and meperidine. The pharmacological actions of these drugs are highly diverse, and the specific mechanisms by which they stop shivering remain unknown. However, they are known to reduce the threshold for shivering, which suggests actions on the central thermoregulatory system rather than in the periphery. Although most opioids significantly impair thermoregulatory control, meperidine is unique, as it is considerably more effective in treating shivering than equianalgesic doses of other µ-agonists. This suggests that the action of this drug is mediated by non–µ-opioid receptors. Meperidine possesses considerable k α opioid receptor activity, as do the mixed agonist-antagonists nalbuphine and butorphanol, and also has central anticholinergic activity. However, neither mechanism appears to mediate the special antishivering activity of meperidine. Instead, this may result at least in part from agonist activity at central α a2-adrenoceptors.18 This action is shared by clonidine, and the central cholinergic activity of physostigmine may act synergistically with these adrenoreceptor subtypes in temperature regulation. Despite confusion regarding precise mechanisms, it is safe to say that these particular drugs are effective and reasonably comparable in their efficacy for managing perioperative shivering.
SUMMARY
Mild hypothermia is extremely common during anesthesia and surgery. The basic process occurs as core body heat redistributes to the skin surface through anesthetic-induced vasodilation and depression of hypothalamic thermoregulatory centers. Heat loss occurs mostly through skin via radiation and convection. A temperature drop of 1°C to 2°C is not uncommon. The physiological effects of hypothermia may have significant potential for detrimental effects on patient well-being. Major consequences of inadvertent hypothermia include morbid myocardial events, reduced resistance to surgical wound infection, impaired coagulation, delayed recovery, and postoperative shivering. Efforts to maintain intraoperative body core temperature higher than 36°C will prevent significant complications, improving the quality and safety of anesthesia care for our patients.
ABOUT THE AUTHORS
Dr. Díaz has a private practice, Advanced Aesthetic Center for Oral and Maxillofacial Surgery, in Weston, Florida.
Dr. Becker is a Professor of Allied Health Sciences at Sinclair Community College and Associate Director of Education, General Dental Practice Residency, at Miami Valley Hospital in Dayton, Ohio.
REFERENCES
1. Sessler DI. Mild perioperative hypothermia. N Engl J Med. 1997;336:1730-1737.
2. Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Philadelphia: Elsevier Inc; 2006:889-901.
3. Sessler DI. Temperature monitoring. In: Miller RD, ed. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier, Churchill Livingstone, 2005:1571-1597.
4. Hanania NA, Zimmerman JL. Hypothermia. In: Hall JB, Schmidt GA, Wood LDN, eds. Principles of Critical Care. 3rd ed. New York: McGraw-Hill; 2005.
5. Morgan GE Jr, Mikhail MS, Murray MJ. Patient monitors. In: Morgan GE Jr, Mikhail MS, Murray MJ, eds. Clinical Anesthesiology. 4th ed. New York: McGraw-Hill Companies Inc; 2006.
6. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277:1127-1134.
7. Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;344:1209-1215.
8. Smied H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289-292.
9. Kurz A, Sessler DI, Narzt E, et al. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7:359-366.
10. Michelson AD, MacGregor H, Barnard MR, et al. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemost. 1994;71:633-640.
11. Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007-1014.
12. Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia: a critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171-201.
13. Hypothermia Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556.
14. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563.
15. Gronert GA, Pessah IN, Muldoon SM, Tautz TJ. Malignant hyperthermia. In Miller RD, ed. Miller’s Anesthesia. 6th ed. Philadelphia: Elsevier, Churchill Livingstone; 2005.
16. Horn EP, Sessler DI, Standl T, et al. Nonthermoregulatory shivering in patients recovering from isoflurane or desflurane anesthesia. Anesthesiology. 1998;89:878-886.
17. Horn EP, Schroeder F, Wilhelm S, et al. Postoperative pain facilitates non-thermoregulatory tremor. Anesthesiology. 1999;91:979-984.
18. DeWitte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467-484.