You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
GLOSSARY
absorbed dose – measure of the energy absorbed per unit mass of matter by any type of ionizing radiation; expressed in rad or Gray units
absorption - transfer of some or all of x-ray photon energy to material or matter; dependent on the energy of the x-ray beam and composition of the absorber
ALARA – safety principle that states that radiation exposure should be kept to minimum or as low as reasonably achievable
atrophy - diminished size or shrinkage in the size of a cell, tissue or organ caused by cell death; a wasting process or progressive degeneration
background radiation – radiation encountered in daily living arising from natural and artificial sources
charge coupled device (CCD) – solid-state, silicon chip detector that converts light or x-ray photons to electrons
collimation – device used to restrict the size and shape of the x-ray beam
complementary metal oxide sensor (CMOS) – solid-state detector similar to the CCD with built-in control functions, smaller pixel size and lower power requirements
coulomb per kilogram – measures the number of electrical charges or ion pairs in a kilogram of air
cross-linking – side spur creation by radiation exposure and attachment to adjacent macromolecule or portion of the same molecule
cumulative effect – additive biologic effect from repeated exposure to radiation
desquamation – ulceration and shedding or loss of the skin
deterministic effect – biologic response whose severity varies with radiation dose; usually a threshold dose exists
detriment – Total harm to health experienced by an exposed group and its descendants as a result of the group’s exposure to a radiation source
direct effect – damage that occurs when ionizing radiation interacts directly with a radiosensitive molecule
dissociation – to disjoin or separate
dose – amount of energy absorbed by an irradiated object
dose rate – dose in rad/Gy absorbed per unit time
dosimeter – device that detects and measures exposure to ionizing radiation
effective dose – the sum of the weighted equivalent doses for the radiosensitive tissues and organs of the body; used to estimate risk, expressed in sieverts
epilation – loss of hair
equivalent dose – mean absorbed dose in a tissue or organ modified by the radiation weighting factor; compares the biologic effect of different types of radiation on tissue; expressed in rem or sievert units
ergs – a unit of work or energy equivalent to 0.624 x 1012 electron volts
excitation – addition of energy to a system through x-ray exposure; result of low energy photon interaction with outer shell electron
erythema – redness of the skin that resembles sunburn due to congestion of the capillaries from high doses of radiation
exposure – measure of radiation quantity; ability of radiation to ionize air by x-rays or gamma rays; expressed in roentgen (R) or Coulomb per kilograms (C/kg)
film badge – photographic film used for personnel monitoring to measure radiation exposure to radiation workers
filtration – removal of the longer wavelength x rays from the primary beam with aluminum or other metal; improves beam quality and reduces patient exposure
free radical – uncharged molecule containing a single unpaired electron in the valence shell
genetic effect – effects produced in reproductive cells that affect future unexposed generations
heritable – capable of being inherited as a genetic trait
image receptor – devices such as film, intensifying screens and digital sensors used to capture a latent image by exposure to x-rays and made visible by chemical, laser or computer processing
indirect effect – radiation effect that results from the interaction of radiation with water and the production of free radicals
ionization – removal of orbital electrons from the atom creating positive and negative ions
kilovoltage – potential difference between the anode and cathode in an x-ray tube; controls the quality or penetrating power of the x-ray beam
latent period – time period between irradiation and the manifestation of an effect
Law of Bergonié and Tribondeau – the radiosensitivity of cells is directly proportional to their reproductive activity and inversely proportional to their degree of differentiation
leakage radiation – form of secondary radiation emitted from the tubehead housing
linear energy transfer – measure of the rate that energy is transferred from ionizing radiation to the tissue
linear nonthreshold response – biologic response that is directly proportional to dose; no threshold dose necessary for damage to occur
macromolecule - a very large molecule with a polymeric chain structure (combination of simpler molecules) such as proteins and polysaccharides
maximum permissible dose – dose of radiation not expected to produce any significant radiation effects
mitotic rate – rate or frequency of somatic cell division or mitosis in which a parent cell divides to form two daughter cells identical to the parent cell
mucositis – radiation-induced redness and inflammation of the mucous membranes
non-stochastic effects – biologic effects of radiation that demonstrate a threshold; increased severity of damage with increased dose
nonlinear threshold dose – varied biologic responses produced by varied doses of radiation with a certain level below which no response occurs
occupational exposure – radiation exposure that is received by radiation workers
osteoradionecrosis – radiation-induced damage and death of bone
photon – electromagnetic radiation in the form of x-rays and gamma rays that interact with matter like a particle or small bundle of energy rather than a wave
photostimulable phosphor plate (PSP) – receptor composed of polyester base coated with a crystalline halide emulsion which converts x-ray energy into stored energy
point lesion – change that causes impairment or loss of function at the point of a single chemical bond as a consequence of irradiation of a macromolecule
position-indicating device (PID) – open-ended cylinder or rectangular device that is inherent or attached to the x-ray tubehead; guides and collimates the primary beam of radiation toward the patient
primary barrier – protective barrier adequate to absorb the primary or useful beam
radiation caries – rampant form of dental decay as a result of radiotherapy and exposure of the salivary glands; altered salivary flow, pH and viscosity hamper buffering and cleansing actions
radiolysis – dissociation or break up of the water molecule as a result of irradiation
radiosensitivity – relative susceptibility of cells, tissues and organs to the effects of ionizing radiation
relative biologic effectiveness – compares the biologic effectiveness of any type of radiation to a test radiation to produce the same effect
rem – stands for roentgen equivalent man; a unit of radiation dose equivalent.
roentgen – x-ray quantity based on the ability of x-rays or gamma rays to ionize air; expressed as R or coulomb per kilogram; named for Wilhelm Conrad Roentgen
scatter radiation – x-rays that have been diverted and scattered back toward the x-ray beam
secondary radiation – scatter and leakage radiation produced as a result of primary beam interaction with matter
secondary barrier – protective barrier adequate to absorb secondary radiation
secondary electron – ejected electron from the outer shell of an atom
side spurs – small, spur-like structures that extend from the main chain macromolecule
sievert – a SI unit for the dosage of ionizing radiation equal to 100 rems
somatic effects – effects of radiation limited to the exposed individual and not passed one to future generations
stochastic effects – the probability of a biologic response to radiation as a function of dose; no threshold dose
thermoluminescent dosimetry – emission of light by a thermally stimulated crystal after radiation; used for occupational and environmental monitoring
threshold dose – dose at which a biologic response first occurs
viscosity – physical property of a substance that is dependent on the friction of the component molecules; a sticky or gummy flow
whole-body exposure – radiation exposure to the entire body rather than a specific or localized area
xerostomia – dryness of the mouth
RADIATION BIOLOGY
Radiation biology is defined as the study of the effects of ionizing radiation on biological systems. Understanding the effects is essential to the safe and effective use of radiation for diagnostic and therapeutic purposes. The human organism is configured in an increasingly complex manner from atoms, to molecules, cells, tissues, organs and systems. Interaction of x-radiation with human tissue occurs at the atomic level through excitation and, more commonly, ionization. (Fig. 1) When an atom is ionized, its chemical binding properties are altered. If the atom is part of a large molecule, ionization may result in breakage of the molecule or a change in location of the atom within the molecule.3 These alterations may impair function and result in cell death. However, cells and tissues can repair, regenerate and recover. Early effects of radiation are injuries that occur within minutes, hours and days while late effects are those injuries that occur within months, years and decades after exposure.
Radiation acts on biologic systems indirectly and directly through the processes of ionization and free radical production. An indirect effect occurs as a result of the radiolysis of water and the production of free radicals while a direct effect occurs when the ionizing radiation interacts directly with a particularly radiosensitive molecule like deoxyribonucleic acid, DNA.4
Indirect Effect of Radiation
Water is the predominant molecule in a biologic system, approximately 80% by weight. As a result, irradiation of water represents the primary radiation interaction in the body.5 Ionization or radiolysis of H2O results in dissociation into two ions or an ion pair (positively charged water molecule and displaced electron). A number of complex reactions can occur after the initial ionization event. The ion pair may reform into a stable water molecule without any damage. On the other hand, if the ions do not rejoin, the negative ion can attach to another water molecule to produce a third type of ion, a negatively charged water molecule.5 As a result, a series of unstable molecules are formed which rapidly dissociate into reactive products called free radicals, largely hydrogen (H*) and hydroxyl (OH*) free radicals. Free radicals are uncharged or neutral atoms with a single unpaired electron in the outer electron shell and, thus, are very reactive. Free radicals contain excess energy that can be transferred to other molecules to disrupt bonds, produce point lesions and form products poisonous to the cell.4 A hydroxyl (OH*) free radical can join with another to form hydrogen peroxide (H2O2) and the hydrogen (H*) free radical can interact with molecular oxygen (O2) to form the hydroperoxyl radical (HO2*). These two products are considered to be the primary damaging agents resulting from the radiolysis of water.4 Other free radicals can be produced by the interaction of hydrogen and hydroxyl free radicals with organic molecules. Organic free radicals are unstable and transform into stable altered molecules that have different chemical and biological properties than the original molecules.6 Since free radicals and altered biomolecules can migrate freely through the tissues, the indirect effects of radiation can manifest at sites distant from the original exposure. The indirect effect accounts for approximately ⅔ of radiation induced biologic damage.
Direct Effect of Radiation
The direct effect occurs when the energy of the photon or secondary electron ionizes a biologic macromolecule such as a protein, lipid, carbohydrate, or nucleic acid.7 Protein and nucleic acid synthesis is essential to cell reproduction and survival. Radiation damage to these macromolecules may result in late effects or cell death. Cells that produce more macromolecules are less radiosensitive. For instance, proteins are more abundant and less radiosensitive while DNA is not very abundant and is the more radiosensitive.8 Because it contains the genetic information for each cell, DNA is the most important macromolecule. The cell nucleus holds the DNA and chromosomes that control growth and development of the cell. Radiation may break DNA strands, alter base sequence, disrupt molecular bonds and cause cross-linking of DNA strands.6
When macromolecules are irradiated, three major effects occur; main-chain scission, cross-linking and point lesions.9 Main-chain scission is a cutting or breakage in the long-chain molecule that divides a long, single molecule into many molecules. This breakage reduces the size of the molecule and changes the viscosity of the macromolecule solution. In cross-linking, irradiation of a macromolecule can produce side spurs that will attach to another segment of the molecule or a neighboring molecule. Although some macromolecules have side spurs, those created by irradiation increase viscosity of the macromolecule solution. In addition, irradiation can produce point lesions. Point lesions occur at the point of a single chemical bond and can cause impairment or loss of cell function. These effects are reversible through repair and recovery. Altered macromolecules differ structurally and functionally from the original macromolecules. The direct effect accounts for ⅓ of radiation induced biologic damage.
Short and Long-Term Effects
The effects of radiation are not evident immediately. There is a delay between irradiation and the appearance of biologic damage. This delay or time interval is known as the latent period. The actual length of the latent period depends on the total dose and delivery rate. Generally speaking, a high dose delivered over a short period of time will result in a brief latent period. Short-term, early or acute effects may occur minutes, hours, or weeks following exposure. Usually short-term effects are the result of high doses of radiation to the whole body. The symptoms include nausea, vomiting, diarrhea, fever, hair loss, hemorrhage and total collapse. The ultimate early effect is death. Long-term, late or chronic effects may occur months, years, or decades following exposure. Long-term or chronic effects are usually the result of low doses of radiation received over a long period of time. These effects may not be observable for months, years and decades and may result in cancers later in life.
The effects of radiation are cumulative or additive. Although tissues have the capacity to repair damage, some damage is not repairable and accumulates in the tissues. The cumulative effect is residual injury without repair from repeated radiation exposure. Low doses received by patients from dental radiography produce very little damage yet some damage does occur. This unrepaired damage may lead to health problems later in life such as cataracts, cancer, leukemia, genetic abnormalities and congenital defects. The critical organ concept designates various tissues as critical organs for radiological health purposes. This concept is based on organ radiosensitivity and potential biologic effects. The critical organs include the lens of the eye, skin, thyroid gland, bone marrow, gonads and fetus.10 The risk of fatal cancers involving the critical organs is minimal. Regardless of the low risk, it is the clinician’s responsibility to keep radiation exposure to a minimum.
Factors that Influence Radiation Effects
There are a variety of other factors that influence the biologic effects of ionizing radiation. The most significant factors will be presented and include radiosensitivity, linear energy transfer, dose factors, area of exposure, somatic and genetic effects and stochastic and non-stochastic effects.
Radiosensitivity
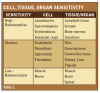
Approximately ten years following the discovery of x-rays, French radiobiologists Jean Bergonié and Louis Tribondeau described the types of cells most sensitive to the effects of radiation. They observed that the most susceptible cells demonstrate a high mitotic rate, long history of cell division, are immature and undifferentiated (not highly specialized) and have a large nucleus to cytoplasm ratio. As a result, some cells, tissues and organs are more sensitive to the effects of radiation than others. Table 1 outlines those tissues that are highly sensitive, moderately sensitive and those that have low sensitivity to ionizing radiation. These observations have become to be known as the Law of Bergonié and Tribondeau.
Linear energy transfer
Linear energy transfer (LET) is the measure of the rate at which energy is transferred from the incident radiation to tissue along the path the radiation is traveling. As the LET increases, so does the biologic damage. This is known as the relative biologic effectiveness (RBE). For example, protons and alpha particles have high LET and are more damaging than x-rays. X-rays have a relatively low LET and are sparsely ionizing. Therefore, they ionize relatively few atoms and/or biomolecules and are less likely to cause a direct biologic effect.
Dose factors
Dose factors have a bearing on the biologic effects of radiation. Dose is defined as the amount of radiation absorbed per unit mass of tissue. Generally speaking, the severity of damage is dependent upon the amount of radiation received. The dose rate is the radiation dose delivered per unit time. The higher the dose rate, the greater the damage done. Lower dose rates allow greater opportunity for repair and less net damage. The total radiation dose can be fractionated or divided into multiple smaller doses delivered over a period of time. This approach is used in radiotherapy as a means of allowing cellular repair and enabling the patient to survive high doses of radiation that are needed to kill tumor cells.
Area of exposure
The volume of tissues exposed to radiation is an important factor as well. The body can tolerate rather high doses (fractionated, 4000-5000 rad/40-50 Gy) to a localized or restricted area of the body. This is one of the principles involved in radiotherapy. One relevant example of a localized area of exposure is therapeutic radiation for head and neck cancer. A variety of tissues would be in the field of radiation such as skin, mucous membranes, taste buds, salivary glands, teeth, muscle and bone. Localized biologic effects could include, erythema, mucositis, changes in taste acuity, xerostomia, radiation caries, muscle atrophy and osteoradionecrosis. On the other hand, high doses of radiation to the whole body are usually fatal. A whole body dose of 100 rad (1 Gy) is usually enough to cause death within several days to weeks.11 Diagnostic x-ray beams are not intense or large enough to cause death but accidental exposure from a nuclear reactor meltdown could produce a lethal whole body dose.11 There are three different syndromes associated with high-level radiation exposure to the whole body, hematological (200–1000 rad/2-10 Gy), gastrointestinal (1000-5000 rad/10-50 Gy) and central nervous system (>5000 rad/50 Gy).12 They are related to the dose received and follow a particular sequence of events called the acute radiation syndrome. There is a prodromal period during which acute clinical symptoms occur followed by a latent period when there is no observable sign of radiation sickness. This is followed by manifest illness, a dose-related period during which the three syndromes occur. The final stage is either recovery or death. If the dose is not lethal, it may take as long as six months for full recovery.12
Somatic and genetic factors
The types of tissues that are exposed to radiation have an influence on the biologic effects of radiation. Generally, the body is divided into two tissue categories, somatic and genetic. Somatic tissues include all tissues of the body except reproductive. The somatic effects of radiation are not passed along to future generations but affect the irradiated individual. Small exposures produce no observable damage but at increasingly higher doses, a threshold will be reached when specific effects are observable. For instance, an individual who receives a localized dose in the 300 to 600 rad (3 to 6 Gy) range will manifest an erythema of the skin within the first or second day.13 After a brief latent period, the erythema reappears followed by skin desquamation and epilation. These types of somatic effects follow a nonlinear, threshold dose-response in which higher doses produce progressively more severe damage. Dental exposures are well below threshold doses and will not produce any observable effects. Genetic tissues include reproductive cells and embryonic tissues. The genetic (heritable) effects of radiation occur only in reproductive cells and can be passed to future generations. These effects do not affect the irradiated individual but rather offspring. These effects include mutation and effects on the embryo that may not be expressed for years or generations. Genetic effects are believed to occur in a linear, nonthreshold dose-response. This means that even small amounts have the potential to produce some mutations. The more the dose increases, the more mutations are produced. Therefore, no dose is considered safe. Gonadal absorbed doses from typical dental radiographic projections rarely, if ever, deliver any measurable dose to the embryo or fetus.14 With patient lead apron protection, the exposure to the reproductive area is virtually zero.15
Stochastic and non-stochastic effects
The biologic effects of radiation can be described as stochastic (chance) and non-stochastic or deterministic. With regard to stochastic effects, the probability of the occurrence of an effect rather than the severity is proportional to the dose. These effects include cancer, mutation and effects on the embryo and do not have dose thresholds. Stochastic effects are all or none; a person either has or doesn’t have the condition.16 The primary concern in dental radiology is cancer rather than reproductive cell mutation or embryonic alterations. With non-stochastic effects, the severity of the response is a function of the radiation dose. A threshold dose must be reached before an effect is observed. Non-stochastic effects occur in all people when the dose is large enough. These effects are proportional to dose, dose rate and the volume of tissues exposed. Dental exposures are well below threshold doses.
Units of Radiation Measurement
There are two different systems of nomenclature for describing radiation quantities, standard or traditional units and SI units (Système International D’Unités.) Traditional units include the roentgen, rad and rem and the SI units include the coulomb/kilogram, gray and sievert. Table 2 outlines the most typical units used to measure quantities of radiation. The roentgen, rad, and rem are considered to be approximately equal to one another.
Exposure
Exposure is a measurement of radiation quantity and refers to the ability of x-rays to ionize air. This is the amount of radiation that is emitted from the x-ray tube and reaches the patient. The measurement is taken at the skin surface before radiation has penetrated the patient’s tissues to measure the intensity of the radiation. The traditional unit for exposure is the roentgen (R) and it measures the amount of x-ray or gamma radiation capable of ionizing 1.6 x 1012 ion pairs per gram of air. Although there is no specific SI unit, 1 roentgen would be equal to 2.58 x 10-4 C/kg of air and 1 C/kg = 3.88 x 103 R (3876 R).
Absorbed dose
The absorbed dose is the measurement of the quantity of any type of ionizing radiation received by a mass of any type of matter. This is the amount of radiation that is actually absorbed by the patient’s tissues. The rad is the traditional unit for the absorbed dose and the gray, the SI unit. One rad is equal to the transfer of 100 ergs per gram of tissue. To convert from traditional to SI units, multiply the rad by 0.01 or multiply the gray by 100 to equal the rad. Absorbed dose units may be expressed in smaller units such as millirad, cGy or mGy.
Equivalent dose
The equivalent dose (HT) is a measurement used to compare the biologic effects or damage an exposed individual might expect to occur from different types of ionizing radiation. The equivalent dose is the mean absorbed dose in a tissue or organ modified by the weighting factor (WT) for the type and energy of the radiation under consideration.14 The weighting factor for x-rays is one, which means that x-rays are less effective in producing biologic effects than alpha particles, which have a weighting factor of 20. The rem (roentgen equivalent man) is the traditional unit and the sievert the SI unit for the equivalent dose. To convert from rem to sievert, multiply the rem by 0.01 or from sievert to rem, multiply the sievert by 100 to equal the rem. The equivalent dose may be expressed in smaller units such as millirem, mSv or μSv (Table 3). The equivalent dose is used to quantify the occupational exposure of radiation workers.
Effective Dose
The effective dose (E) is used to estimate risk or assess biologic consequences in human beings. It is the sum of weighted equivalent doses for the radiosensitive tissues of the body expressed as E = Σ WT x HT. WT is the tissue weighting factor for tissue T and HT is the equivalent dose in tissue organ T.14 The tissue weighting factors vary, for example the weighting factor for the gonads is 0.08 while the weighting factor for thyroid is 0.04.17 The unit of measurement for the effective dose is the sievert (Sv).14
Risk Summary
Dental radiographic x-ray examinations are not without risk. However, the risk is small in terms of other risks readily assumed in daily life that cannot be avoided. Every day humans are exposed to background radiation from the environment. The sources of background radiation include natural and artificial energies and the average annual effective dose is approximately 3.6 mSv. Natural background radiation is the result of radon, cosmic radiation, terrestrial radiation and internal source radiation and accounts for approximately 82% of background radiation exposure. Artificial sources include medical applications such as medical, dental and nuclear medicine diagnostic and therapeutic radiation and consumer products. Artificial sources account for just less than 18% of background radiation exposure. Other sources include occupational radiation, nuclear fuel and nuclear fallout but their contribution is extremely minimal, 0.09%. By way of comparison, a 20-film full mouth survey taken with F speed film and rectangular collimation is equivalent to 1.2 days of background radiation.18
Another way to express risk is the probability of stochastic effects. The primary risk from dental radiography is radiation-induced cancer. The risk of cancer as a result of low dose radiation is very difficult to estimate. Chiefly, risks have been estimated by extrapolation of high dose data from Japanese atomic bomb survivors. Several organizations have estimated that the overall risk of fatal cancers ranges from 8% to 10% Sv-1.19,20,21 These estimates apply to an acute whole body equivalent dose of 1 Sv delivered at a high dose rate and averaged for both sexes and all ages.14 Effects from low level LET radiation like x-rays are dependent on dose and dose rate.14 The ICRP and NCRP adopted a dose and dose rate effectiveness factor of 2 which places risk at 4 to 6% Sv-1.14 However, there is great uncertainty about applying this risk factor to doses less than 100 mSv (0.1 Sv).14 UNSCEAR reported that the average effective dose for an intraoral radiographic examination was 1.3 x 103 mSv and 1.2 x 103 mSv for panoramic radiographic examination.22 These doses are much smaller than the minimum doses for which risk can be determined.14
In 2007, the ICRP published new recommendations which replace those issued in Publication 60 in 1991.17 The 2007 Recommendations update the radiation and tissue weighting factors in the quantities equivalent and effective dose, radiation detriment and the method for calculating effective dose based on the latest scientific information available on the biology and physics of radiation exposure.17 The revised tissue weighting factors include tissues in the maxillofacial region. In 2008, Ludlow et al, studied the impact of the new tissue weighting factors on the effective dose of common dental radiographic examinations and their detriment.23 The results of the study demonstrated increased effective doses for dental radiographic examinations when using the 2007 ICRP calculation method.23 The risks associated with dental radiography were 32% to 422% higher than when using 1990 ICRP guidelines.23 For example, a full mouth survey taken with D speed film and round collimation results in a 20 per million risk of death while the same survey with F speed film or PSP receptors and rectangular collimation reduced the risk ten fold.23 The outcomes of the study suggest an increased possibility of risk with dental exposures and reinforce the need to practice patient dose reduction measures as recommended by the American Dental Association and NCRP.14, 24
In summary, the Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII) concluded that the preponderance of evidence indicates that there is some risk at low doses but the risk is small.25 In addition, the NCRP concluded that radiation risks to dental patients and operators are very small and may be zero.14 However, until there is clear evidence for a threshold dose below which dental patients are not at risk, dental health professionals have an obligation to help patients avoid unnecessary radiation exposure. Methods to reduce radiation exposure to the patient will be discussed in the ongoing text.
SELECTION CRITERIA - GUIDELINES FOR RADIOGRAPHIC EXAMINATIONS
Guidelines for prescribing dental radiographs were first developed by the Food and Drug Administration in 1987 to promote appropriate use of radiographic examinations in dentistry.26 After many years of use, the American Dental Association and the U. S. Department of Health and Human Services updated the document in 2004.27 The underlying principles remain the same but the revised document reflects changes in technology, advances in dental treatment and research outcomes that may influence decision-making. A new category, patients with other circumstances, was added to address imaging needs such as diagnosis of orofacial clinical conditions and to evaluate treatment options.27 Examples of other circumstances may include proposed or existing implants, diagnosis of soft and hard tissue pathology, restorative/endodontic needs, treated periodontal disease and caries remineralization. In addition, practitioners were reminded to attend to the ALARA Principle once the decision to obtain radiographs is made and to use best practices with regard to receptor selection, x-ray beam collimation, exposure and processing techniques and patient shielding (discussed further in the section titled Radiation Safety and Protection).
The guidelines for prescribing radiographs or selection criteria include the following concepts: type of encounter (new or recall), patient age (child, adolescent, adult), stage of dental development (primary, transitional, permanent dentitions; partially edentulous, edentulous), vulnerability to known risk factors (caries, periodontal disease) growth and development monitoring and other circumstances.27 The overarching theme is that the dentist should discuss the medical and dental history with the patient, perform a clinical examination to look for signs or symptoms of disease and determine the necessary radiographs based on the information gathered and the expectation that the radiographs will provide evidence that will affect diagnosis and treatment planning. The use of selection criteria guidelines is a primary concept in reducing patient radiation exposure. Patient examinations should be individualized and only necessary, high yield radiographs should be taken rather than unjustified routine radiographs. Routine, fixed schedules for radiographic examinations for all patients are not recommended except as outlined in the bitewing (horizontal or vertical) guidelines presented below and reduces overall radiation exposure.
There are a number of indicators that the dentist can use to determine the need for dental radiographs. These indicators include the risk for caries, historical findings and positive clinical signs and symptoms. Indicators that would suggest that a patient is at high risk for caries may include evidence of poor oral hygiene, clinical or radiographic caries, recurrent caries, inadequate fluoride exposure, high sucrose diet, radiation therapy and xerostomia. Positive historical findings such as a history of tooth pain or trauma, previous periodontal, endodontic or implant treatment or a family history of dental anomalies may indicate a need for radiographs. In addition, positive clinical signs and symptoms observed during the clinical examination provide evidence that radiographs may be indicated. Examples of positive clinical signs and symptoms include clinical evidence of periodontal disease and/or carious lesions, large or deep restorations, tooth mobility, trauma, unusual eruption patterns and unexplained missing teeth.
The updated guidelines for the selection of patients for dental radiographic examinations are outlined in following text using the framework previously described; type of encounter, patient age and dental development stage, risk factors, growth and development monitoring and other circumstances.
In addition, the following recommendations and considerations should be kept in mind when evaluating the need for dental radiographs. When previous radiographs are available, they should be obtained to determine the need for any further radiographs. All radiographs taken or obtained should be carefully examined for any evidence of caries, bone loss from periodontal disease, developmental anomalies and occult disease. Periodic radiographic examinations solely to screen for occult or hidden pathology in the asymptomatic patient should not be taken. The guidelines need not be altered for patients who are or may be pregnant or patients who have undergone head and neck radiation therapy. When a radiographic examination is indicated, care should be taken to provide protective lead shielding particularly for children, pregnant women and women of childbearing age.27
In summary, the guidelines were developed to serve as an adjunct to the dentist’s professional judgment as to how to best utilize diagnostic imaging for each patient. The dentist must weigh the benefits of taking ra13
diographs against the risk of exposing a patient to x-radiation. The dentist is in the best position to make an informed decision when the patient’s health history, oral status and susceptibility to disease are taken into consideration. The guidelines are intended to be a resource rather than a standard of care or requirement.27
RADIATION SAFETY AND PROTECTION ALARA
The guiding concept in radiation protection is the ALARA principle: As Low As Reasonably Achievable. Dental professionals have an ethical obligation to the patient to minimize exposure and maximize the diagnostic result. The dentist is responsible for prescribing radiographs based on the application of selection criteria. The radiographer who carries out the radiographic procedures must implement the recommended safety precautions and utilize optimal imaging, exposure and processing techniques to ensure that quality results are produced without re-exposure to the patient.
A number of past as well as recent studies have documented deficiencies in radiographic procedures that increase exposure to patients. The most common factors that contribute to increased exposure were improper processing, kilovoltage miscalibration, receptor selection, improper exposure settings and round rather than rectangular collimation.28,29,30 The American Academy of Oral and Maxillofacial Radiology (AAOMR) has published several articles to assist dental practitioners in the assessment of and compliance with recommended practices and safety measures.31,32 The ADA Council on Scientific Affairs published a report which updated the use of dental radiographs.24 These resources can provide the impetus for improving image quality and reducing patient exposure.
Minimizing Patient Exposure
There are a variety of safety and protection measures that can be implemented to reduce exposure to the dental patient and adhere to the ALARA principle.
Image receptors
There are several types of image receptors used in dentistry. (Fig. 2a & 2b) Radiographic film remains a widely used image receptor. The speed of radiographic film represents the sensitivity of the emulsion to x-rays. The faster the film, the less radiation exposure that is required. Letters of the alphabet denote film speed with D, E, E/F and F speed film commercially available. F speed is the fastest film currently available and its use can significantly decrease the exposure (70% compared to D and 20% compared to E) to the patient without diminishing image quality.33 The FDA recommends that film below E speed should not be used for dental radiography because it contributes to greater than necessary patient exposure.33
Digital receptors are replacing film at an ever-increasing rate. Digital receptors include rigid wired or wireless sensors such as the charged-coupled device (CCD) and the complementary metal oxide semiconductor (CMOS) and photostimulable phosphor plate (PSP) or storage phosphor plate (SPP) receptors. Rigid digital receptors are composed of an array of x-ray or light sensitive pixels on a pure silicon chip. The silicon converts absorbed radiation into an electrical charge proportional to the exposure. The charge is converted to a grey scale image with almost immediate image viewing on the computer monitor. Phosphor plates consist of a polyester base coated with a crystalline halide emulsion that converts x-radiation into stored energy. The energy is released as blue fluorescent light when the plate is scanned with a helium-neon laser beam. The emitted light is captured and intensified by a photomultiplier tube and then converted into digital data. There is a short delay between plate exposure, scanning and image viewing on the computer monitor. Digital receptors offer the further potential benefit of reduced exposure and improved work flow. The degree of dose reduction is dependent on the comparison to a particular film speed. It is estimated that digital radiography reduces patient radiation dose by 75% compared with D speed film, 50% compared with E speed film and approximately 40% compared with F speed film.34 The actual amount of exposure reduction achieved with digital receptors is dependent on a number of factors including speed, collimation, technique and retakes. In a systematic review of digital intraoral radiography, Wenzel and Moystad concluded that achievement of patient radiation dose reduction with digital receptors may not hold true in clinical practice.35 The factors that undermined patient dose reduction were the number of images taken, more errors committed and more retakes needed when compared to film. This was particularly true for rigid digital receptors because of their thick, rigid construction, small periphery of dead space reducing the area of image capture and patient discomfort that produced errors causing retakes.35
Regardless of the receptor used, standard infection control measures must be used when taking and processing radiographic images. Digital receptors cannot be sterilized so the clinician must use careful disinfection and barrier coverage techniques to avoid cross-contamination of the receptor.36
Kilovoltage
Dental x-ray machines that operate below 60 kVp result in higher radiation doses to the patient. Low-energy x-rays are absorbed by the patient’s tissues and do not contribute to image production. The acceptable kilovoltage range is between 60 and 80 with optimal settings between 60 and 70 kV.14
Eliminate chemical processing errors
Improper chemical processing techniques and the use of solutions beyond their useful lifespan contribute to poor film-based image quality and result in unnecessary retakes. Proper time and temperature regimens should be followed with daily solution replenishment, processor maintenance and solution change at regular intervals. Implementation of a quality assurance (QA) program to assess processing solutions and darkroom conditions will help eliminate and prevent retakes.14 An important aspect of a quality assurance program is to maintain a log of all QA procedures including date, procedure, results and corrective actions taken.14
There are a variety of waste products associated with film-based radiography. It is best to consult local, state and federal regulations regarding proper disposal of lead foils, spent processing solutions and wash water as well as silver recovery from spent fixer and discarded radiographs.14 Adoption of digital radiography eliminates the darkroom, machine maintenance, hazardous waste disposal and errors associated with chemical processing.14
Patient shields
Thyroid collar: The thyroid gland, particularly in children, is among the most sensitive organs to radiation-induced tumors.14 Even with proper radiographic techniques, the x-ray beam may pass near or through the thyroid.14 In addition, there is some evidence that radiation exposure of the thyroid during pregnancy is associated with low birth weight44 and that dental exposures may contribute to thyroid cancer.45 A thyroid collar should be used on all patients for intraoral radiography, especially children and pregnant women.27 Use of the thyroid collar results in a 50% or greater exposure reduction to the thyroid gland.46 The NCRP recommends use of the thyroid collar for children and adults when it does not interfere with the image study such as in panoramic radiography.14
Lap apron: The use of the lead apron in dentistry has been controversial. The AAOMR states that the gonadal dose from dental radiography is so minimal that use of the lead apron (Fig. 7) should be considered optional unless required by law.32 The updated selection criteria guidelines recommend that a protective thyroid collar and lead apron be used whenever possible and strongly recommends their use when imaging children, women of childbearing age and pregnant women.27 The NCRP recommends that a lead apron need not be provided if all other recommendations in the report are followed rigorously.14 NCRP Report 145 includes a large number of recommendations, 109 total, which would require compliance in order to dispense with lap apron shielding.14,47 The recommendations include the use of: rectangular collimation with specifications, thyroid shielding, fast film or digital intraoral receptors, rare earth intensifying screens with matched film for film-based extraoral radiography or digital extraoral radiography, soft tissue filteration and beam collimation in cephalometric imaging, open-ended PIDs with lengths of 20 cm or longer and other directives related to dental radiology personnel and safety practices.14 Additionally, the dentist is expected to utilize selection criteria in the prescription of patient radiographic examinations, to obtain qualified expert guidance in facility design and radiation protection, to establish office quality assurance and radiation protection program including radiation safety training of staff and continuing education in radiology.14 The majority of these recommendations have been discussed in the preceding text. If any measure is not implemented, then a lead apron should be provided. In summary, the ALARA principle infers that all precautions be taken to keep exposure as low as reasonably achievable. To err on the side of patient protection and dose reduction is prudent and reflects best practice.
Lead shields should have at least .25 mm of lead or lead equivalent. Some states have requirements regarding the thickness of lead or lead equivalent for patient shields. Since lead is a very soft metal, care should be taken when handling or storing lead shields. Avoid bending, creasing or folding shields and use apron hangers to allow the shield to hang flat and properly distribute its weight. The NCRP recommends that patient shields be visually inspected for defects on a monthly basis and more frequently if damaged.14 Further, fluoroscopic examination for hidden defects is recommended on an annual basis 14 As an alternative to lead aprons, light weight lead-equivalent shields are available that utilize materials that effectively absorb scatter radiation but are not as heavy and may be more comfortable for patients and more durable.
Operator Protection Principles
Sources of radiation
There are three sources of radiation in the dental office, primary, secondary and leakage. Primary and leakage radiation emanate from the x-ray machine. Primary radiation is generated at the anode target, collimated by the PID and directed toward patient to produce radiographs. Secondary radiation is scatter radiation created by primary beam interaction with matter such as the patient’s face and oral structures. And leakage, a form of secondary radiation, is emitted from the tube head encasement when x-rays are generated inside the x-ray tube. The dental assistant should avoid all three sources to minimize occupational exposure.
Maximum permissible dose and maximum accumulated dose
Individuals who use radiation to carry out their professional responsibilities are classified as occupationally exposed persons. Dose limits have been established for occupationally exposed persons, nonoccupationally exposed persons (general public) and occupationally exposed pregnant women. The maximum permissible dose (MPD) is the dose of whole body radiation that is not expected to produce any significant somatic or genetic effects in a lifetime. Table 4 outlines the MPD for all three categories in both traditional and SI units and well as a formula for calculating the maximum accumulated dose for occupationally exposed persons. MPD compliance is achieved through adherence to radiation safety practices. The dental assistant’s ultimate goal should be zero occupational exposure.
Minimizing Operator Exposure
Standard rules
Minimizing occupational exposure is not difficult to achieve. The dental assistant should consistently practice standard safety measures and utilize a radiation barrier or the distance and position rule to avoid exposure. In addition, safety measures employed to reduce exposure to dental patients also reduce operator occupational exposure. In simple terms, the dental assistant should avoid the primary beam. The dental assistant SHOULD NOT stand in or near the primary beam or its path, hold the x-ray head or PID in place, hold receptor in the patient’s mouth or hold the patient in position. If assistance is required to stabilize a patient, a parent or guardian should be shielded and restrain the dental patient. If the x-ray head or PID drifts, the unit should be serviced professionally rather than risk occupational exposure.
A study by Kumazawa et al. indicated that the average annual occupational dose for dental personnel was 0.2 mSv.48 Few dental personnel received more than 1 mSv and the vast majority received exposures well below detectable levels.48 The vast majority of dental radiation workers do not require individual monitoring. The NCRP recommends that monitoring of dental personnel be considered for individuals who are expected to receive an annual effective dose in excess of 0.1 rem or 1 mSv and that personal dosimeters be provided for known pregnant occupationally exposed individuals.14 The MPD for a pregnant radiation worker is limited to 0.5 rem or 5 mSv for the duration of the pregnancy and monthly monitoring can help keep occupational exposure below this limit. Any work restrictions for pregnant radiation workers should be based on recommendations by their physician and compliance with institutional policies or state law.14,35 The preceding NCRP recommendations regarding occupational exposure during pregnancy are advisory only.
The U. S. Nuclear Regulatory Commission (NCR) has specific regulations (10 CFR 20.1502) which govern conditions requiring individual monitoring for the declared pregnant women likely to receive during the entire pregnancy from radiation sources external to the body, a deep dose equivalent in excess of 0.1 rem (1 mSv).49 These regulations require that such individuals be instructed in the health issues associated with occupational exposure to radiation including estimated doses and associated risks as described in Regulatory Guides 8.29 and 8.13.50,51 The purpose of providing this instruction is to help pregnant women make informed decisions regarding radiation exposure during pregnancy. The information should be given orally, in printed form or in any other effective communication and the worker should be given an opportunity to discuss the information and ask questions of the supervisor.50 In order for the pregnant worker to take advantage of the lower radiation dose limit and dose monitoring in 10 CFR 20.1502, the woman must declare her pregnancy in writing to the licensee.52 The worker can elect to complete a guide form letter declaring pregnancy and agrees to comply with a radiation dose not to exceed 0.5 rem (5 mSv) during the entire pregnancy with the understanding that meeting the lower dose may require a change in job or job responsibilities during pregnancy.52 If the worker chooses not to declare pregnancy, she will be subject to the radiation dose limits that apply to any other occupational worker.52
SUMMARY
Dental radiographic examinations are not without risk. X-radiation has the potential to damage tissue through either the indirect effect or direct effect of radiation. There are many factors that influence the biologic effects of ionizing radiation such as tissue radiosensitivity, linear energy transfer, dose factors, the volume of tissues exposed to radiation, somatic and genetic effects and stochastic and non-stochastic effects. The biologic effects of radiation are cumulative and every effort must be taken to keep radiation exposures as low as reasonably achievable. A variety of radiation safety and protection measures can be employed to reduce exposure to dental patients and minimize occupational exposure.
ABOUT THE AUTHOR
Gail F. Williamson is a Professor of Dental Diagnostic Sciences in the Department of Oral Pathology, Medicine and Radiology at Indiana University School of Dentistry in Indianapolis, Indiana. She received an A. S. in Dental Hygiene, a B. S. in Allied Health and a M. S. in Education from Indiana University. She serves as Director of Allied Dental Radiology and Course Director for Dental Assisting and Dental Hygiene Radiology Courses. A veteran teacher, Prof. Williamson has received numerous awards for teaching excellence. She is a published author and presents numerous continuing education courses on Oral and Maxillofacial Radiology on the national, regional, state and local levels.
REFERENCES
1. Langland OE and Langlais RP. Early pioneers of oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:496-511.
2. Langland OE and Langlais RP: Principles of Dental Imaging, 2nd Ed., Philadelphia: Lippincott Williams & Wilkins, 2002;311-12.
3. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:451.
4. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:474.
5. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:473.
6. Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 5th Ed. St. Louis: Mosby Inc., 2004:25.
7. Langland OE and Langlais RP: Principles of Dental Imaging, 2nd Ed., Philadelphia: Lippincott Williams & Wilkins, 2002;301.
8. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001: 471.
9. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:470.
10. Langland OE and Langlais RP: Principles of Dental Imaging, 2nd Ed., Philadelphia: Lippincott Williams & Wilkins, 2002:303.
11. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:484.
12. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:485.
13. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:488.
14. National Council on Radiation Protection and Measurements. Radiation protection in dentistry. NCRP Report No. 145. Bethesda, MD. National Council on Radiation Protection and Measurements, 2003.
15. Langland OE and Langlais RP: Principles of Dental Imaging, 2nd Ed., Philadelphia: Lippincott Williams & Wilkins, 2002:309.
16. Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 5th Ed., St. Louis: Mosby Inc., 2004:25.
17. Valentine J. The 2007 Recommendations of the International Commission on Radiological Protection. Oxford, England: Elsevier; 2007.
18. Radiation Safety in Dental Radiography. Rochester, New York. Eastman Kodak Company 2004:4.
19. National Academy of Sciences/National Research Council. Health effects of exposure to low levels of ionizing radiation. Committee on the Biological Effects of Ionizing Radiation (BEIR V). Washington: National Academy Press, 1990.
20. International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. New York: Elsevier Science. ICRP Publications 60, Annals of the ICRP 1991;21(1-3).
21. National Council on Radiation Protection and Measurements. Risk estimates for radiation protection. NCRP Report No. 115. Bethesda, MD. National Council on Radiation Protection and Measurements, 1993b.
22. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and the effects of Ionizing Radiation. Volume 1, Sources, UNSCEAR 2000 Report to the General Assembly, with References. Publication E.00.IX.3. United Nations, New York.
23. Ludlow JB, Davies-Ludlow LE, White SC. Patient Risk Related to Common Dental Radiographic Examinations: Impact of 2007 International Commission on Radiological Protection Recommendations Regarding Dose Calculation. JADA 2008;139;1237-1243.
24. American Dental Association Council on Scientific Affairs. The use of dental radiographs: update and recommendations. JADA 2006;137:1304-12.
25. National Academy of Sciences/National Research Council. Health effects of exposure to low levels of ionizing radiation. Committee on the Biological Effects of Ionizing Radiation (BEIR VII Phase 2). Washington: National Academy Press, 2006. Available at: http://books.nap.edu/catalog/11340.html . Accessed September 12, 2011.
26. U.S. Department of Health and Human Services. The selection of patients for x-ray examinations. Washington, DC, Health and Human Services Publ (Food and Drug Administration) 88-8273, 1987.
27. U.S. Department of Health and Human Services, Public Health Service, Food and Drug Administration and American Dental Association, Council on Dental Benefit Programs, Council of Dental Practice, Council on Scientific Affairs. The selection of patients for radiographic examinations. Rev. ed. 2004. Available at: http://www.ada.org/sections/professionalResources/pdfs/topics_radiography_examinations.pdf. Accessed September 12, 2011.
28. Platin E, Janhom A, Tyndall. A qualitative analysis of dental radiography quality assurance practices among North Carolina dentists. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:115-20.
29. Button TM, Moore WC, Goren AD. Causes of excessive bitewing exposure: Results of a survey regarding radiographic equipment in New York. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:513-17.
30. Yakoumakis EN, Tierris CE, Stefanou EP et al. Image quality assessment and radiation doses in intraoral radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:362-68.
31. Goren AD, Lundeen RC, Deahl ST at al. Updated quality assurance self-assessment exercise in intraoral and panoramic radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:369-74.
32. White SC, Heslop EW, Hollender LG, Mosier KM, Ruprecht A, Shrout MK. Parameters of radiologic care: An official report of the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:498-511.
33. U. S. Food and Drug Administration Center for Devices and Radiological Health. Dental radiography: Doses and film speed. Available at: http://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/NationwideEvaluationofX-RayTrendsNEXT/ucm116524.htm Accessed September 30, 2011.
34. Frederiksen NL: Health Physics. In Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 5th Ed. St. Louis: Mosby Inc., 2004:57.
35. Wenzel A, Moystad A. Work flow with digital intraoral radiography: A systematic review. Acta Odonttologica, 2010;68:106-114.
36. Infection Control in Practice. Dentistry’s Resource for Infection Control & Safety. OSAP chart & checklist: Health and safety considerations for dental radiology. Annapolis, MD. OSAP 2005;4:1-8.
37. Frederiksen NL: Health Physics. In Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 5th Ed. St. Louis: Mosby Inc., 2004:59.
38. Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 6th Ed. St. Louis: Mosby Inc., 2009:37.
39. Frederiksen NL: Health Physics. In Pharoah MJ and White SC: Oral Radiology: Principles and Interpretation, 5th Ed. St. Louis: Mosby Inc., 2004:60.
40. Gibbs SJ. Effective dose equivalent and effective dose: Comparison for common projections in oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:538-45.
41. Parrott LA, Ng SY. A comparison between bitewing radiographs taken with rectangular and circular collimators in UK military dental practices: a retrospective study. Dentomaxillofac Radiol 2011;40(2):102-9.
42. Parks, ET. Errors generated with the use of rectangular collimation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1991;71(4):509-13
43. Zhang w, Abramovitch K, Thames W, Leon IK, Colosi, DC, Goren AD. Comparison of the efficacy and technical accuracy of different rectangular collimators for intraoral radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:e22-e28.
44. Hujoel P, Bollen A-M, Noonan CJ, del Aguila MA. Antepartum dental radiography and infant low birth weight. JAMA 2004;291:1187-93.
45. Memon A, Godward S, Williams D, Siddique I, Al-Saleh K. Dental x-rays and the risk of thyroid cancer: A case-control study. Acta Oncologica 2010:49:447-453.
46. Langland OE and Langlais RP: Principles of Dental Imaging, 2nd Ed., Philadelphia: Lippincott Williams & Wilkins, 2002:322.
47. Preece J. NCRP radiation protection in dentistry – a challenge for the dental profession. AADMRT Newsletter, Winter 2011. Available at: http://www.aaddmrt.com/static.aspx?content=currents/ptreece_winter_11 Accessed September 30, 2011.
48. Kumazawa S, Nelson DR, Richardson AC. Occupational exposure to ionizing radiation in the United States: A comprehensive review for the year 1980 and a summary for the years 1960-1985. Washington. U.S. Environmental Protection Agency; 1984. EPA 520/1-84/005.
49. United States Nuclear Regulatory Commission. NRC Regulations 10 CFR 20.1502 Conditions requiring individual monitoring of external and internal occupational exposure. May 1991. Available at: http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-1502.html. Accessed on September 23, 2011.
50. United States Nuclear Regulatory Commission. Regulatory Guide 8.29 Instruction concerning risks from occupational radiation exposure. February 1996. Available at: http://pbadupws.nrc.gov/docs/ML0037/ML003739401.pdf Accessed on September 23, 2011.
51. United States Nuclear Regulatory Commission. Regulatory Guide 8.13 Instruction Concerning Prenatal Radiation Exposure. Available at: http://www.nrc.gov/reading-rm/doc-collections/reg-guides/occupational-health/rg/8-13/. Accessed on September 23, 2011.
52. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:559.
53. Bushong SC: Radiologic Science for Technologists, 7th Ed., St. Louis: Mosby, 2001:557.