You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Hemangiomas are benign endothelial neoplasms characterized by cellular proliferation.1 They are the most common tumors of infancy and childhood affecting 4% to 10% of Caucasian infants.1 Hemangiomas are three to five times more frequent in females than in males.1-4 A predilection for premature neonates, with 23% incidence in neonates weighing under 1,200 g, has also been pointed out.3,5,6 Fair skin has also hypothesized to be among the risk factors.1,6 Today, there is no universally accepted theory that explains the true etiology and pathophysiology of hemangiomas (such as predilection for the female sex, frequent occurrence after birth, growth and spontaneous involution, abnormal tissue architecture, and so on).4
The majority of hemangiomas, around 80%,2,7 occur on the head and neck. Oral hemangiomas typically appear on the lips, the vermilion border of the lip, buccal mucosa, or on the tongue,2,7 although occasionally they arrive on the palatal or buccal mucosa or gingiva.1 Although benign and mostly do not require surgical intervention, hemangiomas of the lips and tongue can become painfully ulcerated and may bleed. Recurrent hemorrhage, function impairment (problems with eating, speaking, breathing, etc) or poor cosmesis are instances that may require surgical correction.
Treatment
Hemangiomas can be managed in a number of ways, both nonsurgically and surgically. The choice of treatment modality depends on the lesion’s size, location, closeness to other anatomical structures (ie, veins, nerves, etc), and rate of blood flow.8 Nonsurgical management may consist of active observation, corticosteroid therapy (topical or systemic),1 selective embolization, or sclerotherapy as adjunct therapies.9,10 Surgical treatment methods include scalpel excision,1,8 cryosurgery,9,11-13 electrocautery,10 or laser surgery (pulsed dye laser,1 diode,2,14,15 Nd:YAG,2,16,17 or CO210,18-21). A combination of the nonsurgical and surgical methods can be used.
Soft-Tissue Laser Surgery
The key to successful applications of soft-tissue lasers, and their advantages over other surgical tools,8,12,18-26,32-35 is their ability to accurately cut and efficiently coagulate the soft tissue at the same time.
The key to understanding how the laser light cuts and coagulates is through the wavelength-dependent nature of laser light’s interaction with the soft tissue; namely, light absorption and light scattering by the soft tissue.
Laser Light Absorption and Scattering by the Soft Tissue
The absorption depth spectrum in Figure 1 presents the modern understanding22 of how various laser wavelengths interact with the main chromophores (absorption centers) in the oral soft tissue for the three wavelength groups of practical dental lasers that are on the market today:
• circa 1,000 nm (diodes and Nd:YAG laser);
• circa 3,000 nm (Erbium lasers); and
• circa 10,000 nm (CO2 lasers).
Light scattering by the soft tissue is insignificant at Erbium and CO2 laser wavelengths.23-25 Soft-tissue light scattering dominates over absorption at near-infrared diode and Nd:YAG laser wavelengths,22-25 which makes these wavelengths poorly suited for precise ablation, incision, and excision.23-26
Soft-Tissue Laser Ablation
Soft-tissue laser ablation (and incision and excision) is a process of vaporization of intra- and extracellular water heated by the laser light within the irradiated soft tissue.23-25 Water vapors, rapidly steaming out of the intensely laser-heated soft tissue, carry with them cellular ashes and other byproducts of this fast boiling and vaporization process.
Because of weak absorption and strong scattering by the soft tissue, the near-infrared diode and Nd:YAG laser wavelengths circa 1,000 nm are highly inefficient and spatially inaccurate laser ablation tools.23-25 Because of very strong absorption by the soft tissue, mid-infrared Erbium (circa 3,000 nm) and infrared CO2 laser (circa 10,000 nm) wavelengths are highly efficient and spatially accurate laser ablation tools.23-25
Soft-Tissue Laser Coagulation and Hemostasis
Coagulation occurs as the denaturation of soft-tissue proteins occurs in the 60°C to 100°C temperature range, leading to a significant reduction in bleeding as well as oozing of lymphatic liquids on the margins of ablated tissue during laser ablation, excision, and incision procedures. Because blood is contained within and transported through the blood vessels, the diameter of blood vessels (estimated to range from 21 µm to 40 µm, with an average value of 31 µm from measurements in human cadaver gingival connective tissue)27 is a highly important spatial parameter that influences the efficiency of the coagulation process. Collagen shrinks at increased temperatures, which in turn shrinks blood vessel walls and lymphatic vessels, causing hemostasis during laser coagulation.
For Erbium laser wavelengths circa 3,000 nm, optical absorption and coagulation depths are significantly smaller than gingival blood vessel diameters. Coagulation takes place on a relatively small spatial scale and cannot prevent bleeding from the blood vessels severed during tissue ablation. Coagulation depth can be increased by pulse width/rate increase, and by pulse power/fluence decrease.
For diode and Nd:YAG laser wavelengths circa 1,000 nm, optical absorption and coagulation depths are significantly greater than blood vessel diameters. Coagulation takes place over extended volumes—far away from the intended ablation site where no coagulation is required.
For CO2 laser wavelengths circa 10,000 nm, optical absorption and coagulation depths are of the same order as gingival blood vessel diameters. Coagulation extends just deep enough into a severed blood vessel to stop the bleeding. Coagulation depth can be increased by an increase in pulse width/rate, and by decreasing pulse power/fluence.
Optimal Wavelength for Soft-Tissue Laser Surgery
Wavelengths circa 10,000 nm are >1,000 times superior to wavelengths circa 1,000 nm for soft-tissue ablation, and >10 times superior to wavelengths circa 3,000 nm for soft-tissue coagulation and hemostasis. The wavelengths circa 10,000 nm (eg, the CO2 laser) deliver both soft-tissue ablation and simultaneous coagulation, which is unobtainable with either diodes (circa 1,000 nm) or Erbium (circa 3,000 nm) wavelengths.
Laser Pulsing and Thermal Relaxation Time
The application of laser energy over an extended period of time may result in inefficient tissue cutting because of thermal diffusion of laser-generated heat from the irradiated tissue, which may lead to undesirable tissue necrosis and charring on the margins of the laser incision. Proper laser pulsing is of the utmost importance for the appropriate application of laser energy for soft-tissue laser ablation and coagulation.23-25 The most efficient heating of the irradiated tissue takes place when the duration of the laser pulse is much shorter than the thermal relaxation time (which is laser-wavelength and tissue-type specific—Figure 1); the most efficient tissue cooling takes place if the duration between laser pulses is much greater than the thermal relaxation time.
The so-called “SuperPulse” design24 for CO2 laser pulsing parameters is optimized around the thermal relaxation time concept discussed above, and provides for the char-free soft-tissue ablation, incision, and excision with variable depths of coagulation/hemostasis on the margins of the cut.
The SuperPulse mode is made with bursts of short laser pulses with very high peak power that are spaced far enough apart for efficient tissue cooling between the pulses. The SuperPulse minimizes the amount of heat diffusing from the cutting/ablation zone to the surrounding tissue.
Nd:YAG Soft-Tissue Ablation and Coagulation
The Nd:YAG laser’s 1,064-nm wavelength is an efficient coagulator but a poor scalpel, as it is highly scattered and weakly absorbed by the soft tissue.23-25 To enhance its cutting efficiency, the low absorption of the Nd:YAG wavelength can be attenuated by the use of very high peak power24 typical for free-running pulsed Nd:YAG lasers. When used in contact mode, the Nd:YAG laser may function as a hot-tip cutting tool.23-24
CO2 Laser Soft-Tissue Surgery
The CO2 laser is used directly to photo-thermally cut, ablate, and, at the same time, photo-thermally coagulate the soft tissues. The key to the success of the CO2 laser is its ability to cut and coagulate the soft tissue simultaneously. Numerous studies8,18-19 emphasize the CO2 laser’s ability to coagulate (shrink collagen in) blood vessels of up to 0.5 mm, calling it the greatest advantage of this surgical tool. They conclude that the CO2 laser is well suited for vascular lesions (ie, hemangiomas, venous lakes, smaller angioectasias, varicosities, and so on) because the vasculature that supplies blood to these lesions is coagulated, thus facilitating the process of cutting or ablation. The excellent hemostatic capacity of the CO2 laser is described as a useful instrument for oral surgery in patients with blood coagulation disorders or undergoing antithrombotic therapy,8,18,19,28,29 which made the CO2 laser the ideal tool for the case described in this article.
The clinical literature suggests that the use of the CO2 laser for the treatment of vascular lesions, including hemangioma of the lip, presents several advantages over conventional scalpel surgery. Among these advantages are excision18,20,21 or ablation8,19,30,31 without direct tissue contact (hence no mechanical trauma to the tissue) and without bleeding or the need for sutures, precise tissue removal, and minimized postoperative pain and edema. For example, in the study carried out by Apfelberg et al,20 participants reported that “postoperative pain and edema were virtually nonexistent.” The use of a CO2 laser on the oral soft tissue has no known contraindications or side effects.29
Laser Handpieces
Since the early days of surgical CO2 lasers in the 1970s and 1980s, the articulated arm beam delivery system was a barrier to wide adoption of the technology. The paradigm change for CO2 laser surgery was brought about by the invention of the hollow waveguide flexible fiber CO2 laser beam delivery system in the late 1980s. The modern flexible-fiber CO2 laser handpiece is pen-sized, disposable-free, autoclavable, and easily adaptable to switching back and forth between (1) incision with photo-coagulation; (2) superficial ablation with photo-coagulation; and (3) photo-coagulation. Tip-retainer laser handpieces use disposable hollow focusing tips made of high-temperature resistant aluminum-oxide ceramic with a 250-µm spot size to allow for intrasulcular periodontal applications.
Laser Power Density
Consider a steel blade: Regardless of how sharp the blade is, there will be no interaction between the blade and the tissue unless mechanical pressure is applied to the blade, forcing it through the tissue’s surface. For a CO2 laser scalpel, the power density of the focused laser beam is equivalent to the mechanical pressure that is applied to a cold steel blade—the greater the laser power density, the greater the rate of soft-tissue removal (Figure 2 and Figure 3).
Pogrel et al32 observed a relatively narrow, <100-µm, zone of thermal necrosis and a variable zone of reversible thermal changes adjacent to the zone of necrosis (100 µm to 500 µm wide). The authors pointed out that the thermal side effects of the CO2 laser could be controlled and reduced by changing the power setting, pulse duration, and pulse repetition rate. A <50-µm thin coagulation zone thickness was reported by Wilder-Smith et al33 for the flexible fiber CO2 laser at 10,600 nm.
Wound Healing
The ability to provide excellent hemostasis is especially valuable as it allows for more precise and accurate tissue removal because the clinician has improved visibility of the surgical field.19 Because of the hemostatic ability of the CO2 laser, intraoral surgical wounds often can be left to heal by secondary intention without placing sutures or dressing.19,21
Another advantage of the CO2 laser is minimal postoperative swelling and edema because of the intraoperative closure of lymphatic vessels on the margins of the CO2 laser incision. Lymphatic vessels regenerate successively in approximately 8 to 10 days after capillary-vessel proliferation.21
Among the most important advantages of CO2 laser treatment are significantly reduced wound contraction and scarring.18,19,34,35 In CO2 laser-irradiated wounds, the healing process is characterized by a more prominent fibroblastic proliferation, with young fibroblasts actively producing collagen; only a small number of myofibroblasts (the cells responsible for wound contraction) is found in the CO2 laser-excised wounds compared with scalpel wounds.35-37 According to Basu et al,38 and Tambuwala et al,39 healing of the wounds caused by the CO2 laser involves the appearance of a fibroserous membrane 72 hours postoperatively. This membrane replaces the superficial necrotic layer of the laser-treated area. An epithelial covering of the wound starts to form from the periphery toward the center after 2 weeks, and is thinner and parakeratotic in comparison with the epithelium that forms after scalpel resection. This could account for the excellent esthetic outcome of all CO2 laser treatment, with no fibrosis or scarring and soft pliable residual tissue, while a scalpel can leave some scarring.18
Reduced wound contraction combined with decreased lateral tissue damage, less traumatic surgery, more precise control of the depth of tissue damage, and excellent hemostatic ability make the CO2 laser a safe and efficient alternative to the conventional scalpel. Strauss et al19 and Deppe et al40 report that the healing process with CO2 laser surgery is faster and less painful than after cryosurgery or electrosurgery.
The slight disadvantage of the CO2 laser in comparison with surgical scalpel wounds is that the healing process for laser wounds may be prolonged. This delay is believed to be caused by the sealing of blood vessels and lymphatics that subsequently requires neovascularization for healing. Typical intraoral healing takes 2 to 3 weeks for wounds that, if treated with a scalpel, normally would take 7 to 10 days.19,40 At the same time, Lambrecht et al18 reported a slightly shorter delay of just 3 to 10 days. The key to minimizing the healing time is through minimizing the thermal damage on the margins of laser incision/ablation (eg, utilizing the SuperPulse CO2 laser settings).
CO2 Laser Ablation of Hemangioma of the Lip: Case Report
Initial Findings
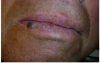
A 68-year-old man presented with a 5-mm x 5-mm nodule on his lower lip. The lesion was located mostly within the vermilion border of the lower right lip, slightly crossing the vermilion-cutaneous junction (Figure 4). Clinically, the nodule appeared to be slightly exophytic, smooth-surfaced, and bluish-purple in color. The patient indicated that it was never painful or irritated, but it had grown. The mass was diagnosed as a hemangioma due to its color, texture, and compressive nature. Cavernous hemagiomas are extremely diffuse and can spread over large areas of tissue.
Diagnosis and Treatment Plan
The patient had had the lesion for as long as he could remember and wanted it to be removed. It was mostly a cosmetic concern, but since he had the lip-biting habit the lesion increased in size. He took anticoagulants for atrial fibrillation, which increased the risk of bleeding if he accidentally bit the lip too hard.
Removal of the lesion, therefore, was challenging for the surgeon for two reasons: (1) its location in the esthetic zone and (2) the potential for intraoperative hemorrhage due to the vascularity of the lesion and the blood thinners this patient had been taking.
The lesion was clinically diagnosed as a hemangioma. It was well circumscribed, soft to palpation, with no pulsation or bruit present. Considering the aforementioned challenges, it was decided to surgically remove the hemangioma utilizing the CO2 laser ablation and coagulation techniques.
Surgical Laser Equipment and Settings
We used the flexible fiber hollow waveguide CO2 laser (LightScalpel LS-1005) with an angled tipless autoclavable handpiece. The handpiece was held 1 mm to 1.5 mm away from the target tissue for ablation and 3 mm to 4 mm away for coagulation. The surgeon’s hand was constantly moving to ensure efficient ablation and excellent hemostasis with minimal thermal damage. Laser settings were as follows:
Power Output: 4 W
Laser Mode: SuperPulse (SP) with repeat pulsing at 15 msec pulse-width and 29 Hz repetition rate
Spot Diameter: 0.25 mm
CO2 Laser Procedure
Anesthesia:
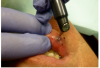
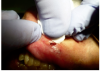
Topical anesthetic was applied to the lesion (Figure 5). Local anesthesia with 2% lidocaine with a 1:100,000 epinephrine mixture was administered by infiltration (Figure 6). Note bleeding at the injection sites in Figure 6 and Figure 7. This reminded the surgeon to be especially careful and mindful of the patient’s condition, which required anticoagulants.
Technique:
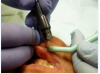
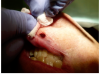
To achieve the optimal laser-tissue interaction, the laser handpiece was held strictly at a 90° angle to the lesion (Figure 7 and Figure 8).
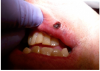
First, the laser beam was used to puncture the lesion in the middle, which caused it to bleed (Figure 8). After puncturing, the contents of the lesion were expressed and its volume decreased (Figure 9). The blood was repeatedly wiped off with 2 x 2 inch sterile gauze pads.
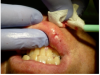
The lesion was gradually ablated from the center outward in a circular spiral motion (Figure 10). Between laser passes, debris was removed with sterile gauze pads to avoid tissue overheating (Figure 11). Ablation continued until the base of the lesion was reached.
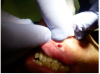
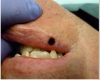
After ablation was completed, the inner surface of the lesion was coagulated with the laser beam (Figure 12). In order to achieve hemostasis, the surgeon defocused the laser beam by increasing the tip-to-tissue distance from 1 mm-1.5 mm to 3mm-4 mm. Despite the vascular nature of capillary hemangioma and the patient’s anticoagulant medication, the laser easily achieved hemostasis (Figure 13). A thin carbonized layer was then created over the wound (Figure 14). It is actually recommended to preserve some carbonization of the surface of the wound in order to reduce the risk postoperative bleeding.31
To minimize any chance of scarring, the wound was left unsutured allowing it to heal by secondary intention. There is considerable body of clinical literature that describes the histology of the tissue-healing process after the CO2 laser surgery which accounts for the lack of scarring—see the section Wound Healing of this article.
Postoperative Instructions
No postoperative care was required other than placement of Vitamin E gel two to three times daily for 2 weeks. The patient was prescribed Percocet for possible pain relief, but did not have to use it; he did take one dose of ibuprofen 600 mg after the procedure. The patient was instructed to avoid abrasive food for 1 week. Secondary intention healing occurred. No sutures were needed.
Follow-Up Evaluation
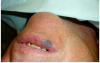
The patient came in for a check-up 1 month after the laser surgery. The patient did not report any pain or edema. No signs of inflammation were present. As expected, healing of the surgical site progressed remarkably well, without any scar formation (Figure 15). The significance of such an outcome is especially high due to the location of the lesion in the esthetic zone.
Discussion
A slightly different technique of the surgical CO2 laser treatment of the hemangioma of the lip is described in Sawich8 and Namour.31 Instead of puncturing the lesion, the laser is defocused and used to dehydrate the hemangioma first, moving from the periphery toward the center. Once the surface appears whitish, the ablation starts. In between laser passes, the area is debrided with a moist gauze pad to facilitate laser energy penetration. Once the ablation is finished, the laser is defocused more and the hemangioma’s bed is coagulated from the center outward to contract the surgical wound. A thin layer of carbonization serves as a physiological bandage. The follow-up photographs show good healing without scarring.
Both of the aforementioned techniques demonstrate excellent outcomes of the CO2 laser treatment of the hemangioma of the lip. Speed and ease of the treatment along with predictable penetration depth and excellent hemostatic properties comprise the main advantages of the CO2 laser treatment of a hemangioma of the lip.
Other Surgical Treatment Modalities
Cryosurgery
Some literature names cryosurgery the preferred method of hemangiomas of the lip removal.9,11,12 Cryosurgery presupposes the lesion destruction by quick freezing in situ. Extremely cold temperatures are applied to the lesion to ensure local tissue destruction (cellular death) and the resulting necrotic tissue is left to slough spontaneously.9,11 The freezing agent, for example, liquid nitrogen, is delivered by direct application to the lesion with a cotton wool bud or spray (open method); or via metal “cryoprobes” (closed method).11 Cremer13 recommends the closed method of cryogen delivery for treating hemangiomas. It should be noted that such treatment is specifically limited to singular superficial, non-complicated or “harmless” hemangiomas with regular circumference.13 Cryosurgery has a number of advantages that made it beneficial for the removal of such vascular lesions as hemangioma, ie, bloodless treatment and low chances of secondary infection, pain, or hemorrhage. Complete healing is achieved within approximately 3 to 4 weeks of treatment. In addition, it is fairly inexpensive and easy. However, this treatment has a number of disadvantages, which include unpredictable depth of tissue necrosis with the possibility of scarring, which is especially problematic if the vermilion border of the lips requires treatment.15,16 In other words, cryosurgery does not allow control of the depth of tissue removal with high precision to the extent that laser surgery does. Other potential complications include pain during freezing, edema and exudation, vesicles, and bullae. Finally, cryosurgery can result in pseudoepitheliomatous hyperplasia, postoperative infection, fever, and pyogenic granuloma.41 Typically, a single session is enough, but in certain cases, several freeze-thaw sessions may be needed.12
Pulsed Dye Laser Photocoagulation
The pulsed dye laser (PDL) has been named the criterion standard for treating vascular lesions.42 The wavelength of 585 nm and 595 nm PDL causes targeted photocoagulation of the blood vessels, leaving the overlying skin undamaged.42 However, Zheng et al point out that due to its shallow penetration depth of approximately 1 mm, the PDL is indicated only for the treatment of superficial and residual vascular lesions (including hemangiomas) and are not effective on subcutaneous and deep hemangiomas. The existing literature also names several possible adverse effects of the PDL treatment, such as subsequent pain, purpura (lasting for 10 days), crusting, risk of atrophic scarring, and painful ulceration.43,44 Kessels et al42 carried out a study that led them to the conclusion that adding epidermal cooling and having longer pulses can reduce the risk of adverse effects.
Soft-Tissue Diode Photocoagulation
Another surgical modality used for treating hemangioma and other vascular lesions is forced dehydration with induced photocoagulation by 810-mm to 980-nm diode laser in the continuous wave mode at 2 W to 4 W.2,14 The laser is used in a non-contact mode—the tip is held approximately 1 cm away from the lesion. The laser energy induces coagulation on hemoglobin—its main chromophore.2 Because of the intense heat generated by the diode laser, a simultaneous air cooling is necessary to reduce damage to the lateral healthy tissues and reduce intraoperative sensitivity.2 The hemangioma lesion becomes whitish within a few seconds. Minimal pain and swelling lasting 1 to 2 days were reported.14 Necrotic tissue sloughing occurs 2 to 3 days after the treatment.14 The treated area typically re-epithelializes and heals within 2 to 3 weeks.14
Nd:YAG Photocoagulation
Nd:YAG lasers are also suitable for the treatment of oral and peri-oral hemangiomas and other vascular lesions,2,16 especially with a 600-µm fiber used with long pulses of 30 to 60 milliseconds.2 The necessity of a dynamic cooling device has been emphasized. The Nd:YAG laser wavelength of 1,064 nm is mostly absorbed by oxyhemoglobin.17 The laser energy penetrates deep into the tissue and causes coagulation up to 7 mm to 10 mm deep, which makes Nd:YAG lasers effective for the treatment of deeper vascular lesions.17 The necrotic tissue is left to slough (within 1 to 3 days) and complete healing is achieved within 3 to 5 weeks.17 In the study conducted by Vesnaver et al,17 moderate pain and discomfort were reported to persist in the first 7 to 10 days after the treatment. In addition, postoperative edema and the possibility of scarring is one of the main adverse effects of Nd:YAG treatment.17 The significant depth of photocoagulation induced by Nd:YAG is not appropriate for superficial lip hemangioma, as in the case described in this article.
Electrocautery
Electrocautery, or thermal cautery, is a process in which a direct or alternating current passes through a resistant metal wire electrode, generating heat. The heated electrode is then applied to living tissue to achieve hemostasis or varying degrees of tissue destruction.45 In electrocautery, unlike electrosurgery, the electric current does not pass through the patient; therefore, the technique can be safely applied with patients with pacemakers, ICDs, and deep-brain stimulators.46 Although electrocautery has been used to treat hemangiomas, there is literature advocating against it in dentistry because of the excessive thermal necrosis, depigmentation, and hypertrophic scarring as its adverse effects.14
Scalpel Excision
Small vascular lesions can be surgically excised with the scalpel.8 The possible complication of such treatment, however, is intraoperative bleeding. In the case described in this article, this risk of bleeding was elevated by the anticoagulants the patient had been taking for his atrial fibrillation condition. Therefore, scalpel excision was not selected for this case.
Conclusion
Hemangiomas always present a challenge for a dental surgeon due to their vascular nature and may pose a serious bleeding risk. Although various methods have been used to treat hemangioma of the lip, we consider the CO2 laser to be the instrument of choice because of its numerous intra- and postoperative benefits: (1) the ability to effectively coagulate small blood vessels allows surgeons to operate on patients who are on anticoagulating medications and provides good visibility during the surgery; it also makes the removal of soft tissue more precise; (2) the non-contact operating mode ensures less mechanical trauma to the tissue and reduces the possibility of wound contamination; (3) excellent esthetic outcome is especially important in areas where cosmesis is a concern.
References
1. Marler JJ, Mulliken JB. Current management of hemangiomas and vascular malformations. Clin Plast Surg. 2005;32(1):99-116.
2. Olivi G, Margolis F, Genovese MD. Pediatric Laser Dentistry: A User’s Guide. Hanover Park, IL: Quintessence Publishing Co, Inc.; 2011.
3. Bolognia JL, Schaffer JV, Duncan KO, Ko CJ. Infantile hemangiomas and vascular malformations. In: Dermatology Essentials. Oxford: Saunders; 2014:812-833.
4. Bauland CG, van Steensel MA, Steijlen PM, et al. The pathogenesis of hemangiomas: a review. Plast Reconstr Surg. 2006;117(2):29e-35e.
5. Amir J, Metzker A, Krikler R, Reisner SH. Strawberry hemangioma in preterm infants. Pediatr Dermatol. 1986;3:331-332.
6. Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291-294.
7. Eivazi B, Ardelean M, Baumler W, et al. Update on hemangiomas and vascular malformations of the head and neck. Eur Arch Otorhinolaryngol. 2009;266(2):187-197.
8. Sawisch TJ. Oral surgery for the general practitioner: ablation/vaporization techniques and procedures—clinical scenarios. In: Convissar RA. Principles and Practices of Laser Dentistry. St. Louis, MO: Mosby; 2011:93-113.
9. Tal H. Cryosurgical treatment of hemangiomas of the lip. Oral Surg Oral Med Oral Pathol. 1992;73(6):650-654.
10. Greene AK. Management of hemangiomas and other vascular tumors. Clin Plast Surg. 2011;38(1):45-63.
11. Kujan O, Azzeghaiby SN, Tarakji B, et al. Cryosurgery of the oral and peri-oral region: a literature review of the mechanism, tissue response, and clinical applications. J Investig Clin Dent. 2013;4(2):71-77.
12. Moritz A. Oral Laser Application. Berlin: Quintessenz Verlags-GmbH; 2006.
13. Cremer H. Cryosurgery for hemangiomas. Pediatric Dermatol. 1998;15(5):410-411.
14. Azavedo LH, Galletta VC, de Paulo Eduardo C, Migliari DA. Venous lake of the lips treated using photocoagulation with high-intensity diode laser. Photomed Laser Surg. 2010;28(2):263-265.
15. Angiero F, Benedicenti S, Romanos GE, Crippa R. Treatment of hemangioma of the head and neck with diode laser and forced dehydration with induced photocoagulation. Photomed Laser Surg. 2008;26:113-118.
16. Bekhor PS. Long-pulsed Nd:YAG laser treatment of venous lakes: report of a series of 34 cases. Dermatol Surg. 2006;32:1151-1154.
17. Vesnaver A, Dovsak DA. Treatment of large vascular lesions in the orofacial region with the Nd:YAG laser. J Craniomaxillofac Surg. 2009;37(4):191-195.
18. Wlodawsky RN, Strauss RA. Intraoral laser surgery. Oral Maxillofac Surg Clin North Am. 2004;16(2):149-163.
19. Strauss RA, Fallon SD. Lasers in contemporary oral and maxillofacial surgery. Dent Clin North Am. 2004;48(4):861-888.
20. Apfelberg DB, Maser MR, Lash H, White DN. Benefits of the CO2 laser in oral hemangioma excision. Plast Reconstr Surg. 1985;75(1):46-50.
21. Lambrecht JT, Stübinger S, Hodel Y. Treatment of intraoral hemangiomas with the CO2 laser. J Oral Laser Appl. 2004;4:89-96.
22. Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58(11):R37-R61.
23. Fisher JC. Basic laser physics and interaction of laser light with soft tissue. In: Shapshay SM. ed. Endoscopic Laser Surgery Handbook. New York, NY: Marcel Dekker; 1987:96-125.
24. Fisher JC. Qualitative and quantitative tissue effects of light from important surgical lasers. In: Wright CV, Fisher JC, eds. Laser Surgery in Gynecology: A Clinical Guide. Philadelphia, PA: Saunders; 1993:58-81.
25. Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103(2):577-644.
26. Willems PWA, Vandertop WP, Verdaasdonk RM, et al. Contact laser-assisted neuroendoscopy can be performed safely by using pretreated ‘black’ fibre tips: Experimental data. Lasers in Surgery and Medicine. 2001;28(4):324-329.
27. Yoshida S, Noguchi K, Imura K, et al. A morphological study of the blood vessels associated with periodontal probing depth in human gingival tissue. Okajimas Folia Anat Jpn. 2011;88(3):103-109.
28. Santos-Dias A. CO2 laser surgery in hemophilia treatment. J Clin Laser Med Surg. 1992;10(4):297-301.
29. Gama SK, De Araujo TM, Pinheiro AL. Benefits of the use of the CO2 laser in orthodontics. Lasers Med Sci. 2008;23:459-465.
30. McClellan AC, Walls SL, Gibson TM, et al. Surgical and laser treatment of hemangiomas of the lips. J Periodontol. 2013 Nov [Epub ahead of print] <http://www.joponline.org/doi/abs/10.1902/cap.2013.130070> Accessed February 18, 2015.
31. Namour S. Atlas of Current Oral Laser Surgery. Boca Raton, FL: Universal Publishers. 2011;139-171.
32. Pogrel MA, McCracken KJ, Daniels TE. Histologic evaluation of the width of soft tissue necrosis adjacent to carbon dioxide laser incisions. Oral Surg Oral Med Oral Pathol. 1990;70(5):564-568.
33. Wilder-Smith P, Arrastia AM, Liaw LH, Berns M. Incision properties and thermal effects of three CO2 lasers in soft tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):685-691.
34. Zeinoun T, Nammour S, Dourov N, et al. Myofibroblasts in healing laser excision wounds. Lasers Surg Med. 2001;28:74-79.
35. Grbavac RA, Veeck EB, Bernard JP, et al. Effects of laser therapy in CO2 laser wounds in rats. Photomed Laser Surg. 2006;24(3):389-396.
36. Fisher SE, Frame JW, Browne RM, Tranter RMD. A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch Oral Biol. 1983;28(4):287-291.
37. de Freitas AC, Pinheiro AL, de Oliveira MG, Ramalho LM. Assessment of the behavior of myofibroblasts on scalpel and CO2 laser wounds: an immunohistochemical study in rats. J Clin Laser Med Surg. 2002;20(4):221-225.
38. Basu MK, Frame JW, Rhys Evans PH. Wound healing following partial glossectomy using the CO2 laser, diathermy and scalpel: a histological study in rats. J Laryngol Otol. 1988;102(4):322-327.
39. Tambuwala A, Sangle A, Khan A, Sayed A. Excision of oral leukoplakia by CO2 lasers versus traditional scalpel: A comparative study. J Maxillofac Oral Surg. 2014;13(3):320-327.
40. Deppe H, Horch HH. Current status of laser applications in oral and cranio-maxillofacial surgery. Med Laser Appl. 2007;22(1):39-42.
41. Faber WR. Side effects and complications in cryosurgery. Dermatol Monatsschr. 1993;179:247-251.
42. Kessels JP, Hamers ET, Ostertag JU. Superficial hemangioma: pulsed dye laser versus wait-and-see. Dermatol Surg. 2013;39(3 Pt 1):414-21.
43. Witman PM, Wagner AM, Scherer K, et al. Complications following pulsed dye laser treatment of superficial hemangiomas. Lasers Surg Med. 2006;38(2):116-123.
44. Batta K, Goodyear HM, Moss C, et al. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1-year analysis. Lancet. 2002;360(9332):521-527.
45. Pollock SV. Electrosurgery. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby Elsevier; 2008: Ch 140.
46. Weaver J, Kim SJ, Lee MH, Torres A. Cutaneous electrosurgery in a patient with a deep brain stimulator. Dermatol Surg. 1999;25(5):415-417.