You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Guidelines for monitoring during anesthesia were first introduced by Eichhorn et al in 1986 when they published guidelines adopted at Harvard Medical School.1 The American Dental Society of Anesthesiology should be credited with its timely follow-up by publishing guidelines for the dental profession in 1991.2 The authors, Drs. Morton B. Rosenberg and Robert L. Campbell, spearheaded a consensus of guidelines that clearly detail appropriate monitoring for moderate (conscious) sedation compared with that for deep sedation and general anesthesia. Unfortunately, the dental profession was slow to appreciate the significance of this publication, and various organizations chose to embark separately on developing guidelines for their particular specialty interest. Today the American Dental Association and many specialty groups in dentistry4 and medicine, for that matter4 have developed and published monitoring guidelines for their members. Fortunately, they are similar in most respects, and they all adhere to the principles suggested currently by the American Society of Anesthesiologists for nonanesthesiologists.3
All guidelines for monitoring respiratory function have in common the following protocols: a) Oxygenation must be monitored continuously by pulse oximetry when providing moderate sedation, deep sedation, or general anesthesia. b) Ventilation must be monitored continually (periodically) during moderate sedation and continuously (uninterrupted) during deep sedation and general anesthesia.
The conventional approach of most courses in pulmonary physiology is to address four categories of events that comprise respiration: ventilation, gas exchange, gas transport, and control of ventilation.4 We will depart from this convention to better correlate physiological concepts with principles of monitoring ventilation and oxygenation.
Ventilation (breathing) and oxygenation are related but separate physiological processes. Ventilation refers to movement of gas between the environment and pulmonary alveoli. Oxygenation refers to the actual oxygen content of arterial blood, and it is determined by adequate ventilation and perfusion of the pulmonary capillaries. When arterial oxygen content is low, the condition is described as hypoxemia and may be attributable to either respiratory or circulatory causes. The terms hypoxia and hypoxemia are frequently used interchangeably, but they are not synonymous. Hypoxia is a more inclusive term referring to diminished oxygen in inspired gas or any tissue, including blood. In the setting of sedation and anesthesia, hypoxia from hypoventilation is the most likely cause of hypoxemia. However, when ventilation is adequate, hypoxemia can also occur if perfusion of the pulmonary alveoli is inadequate. This would be rare in dental practice unless a patient experiences acute heart failure, pulmonary embolus, or cardiac arrest.
OXYGENATION: PHYSIOLOGICAL CONSIDERATIONS
The most essential function of the respiratory system is to deliver oxygen to arterial blood so it can be provided to body tissues. Approximately 98% to 99% of the total oxygen content in arterial blood is bound to hemoglobin in the red blood cells. The remaining 1% to 2% is dissolved in plasma, producing a gas pressure referred to as arterial oxygen tension (PaO2). It is this unbound oxygen that enters the body tissues, driven by the PaO2 gradient. As dissolved oxygen enters the cells, it is replaced instantaneously by oxygen released from hemoglobin. Therefore, there is a relationship between the degree to which hemoglobin is saturated with oxygen (SaO2) and the tension produced by dissolved oxygen (PaO2). This relationship is illustrated by the oxygen-hemoglobin dissociation curve (see Figure 1). Under normal conditions, hemoglobin in arterial blood remains ~98% saturated and sustains a PaO2 of ~95 mm Hg. At the tissue level, things change. Oxygen is delivered to the tissues and oxygen tension declines to ~40 mm Hg with a corresponding hemoglobin saturation of approximately 70% to 75%, normal values or venous blood.
Normal oxygenation is defined as a PaO2 of 80 mm Hg to 100 mm Hg. In critical care medicine, PaO2 can be assessed by sampling arterial blood gases. The introduction of pulse oximetry allowed for a continuous, noninvasive method to approximate PaO2 indirectly by measuring percentage of hemoglobin saturation. The measurement is made using a finger probe; hemoglobin saturation is abbreviated as SpO2 to distinguish it from saturation determined by arterial blood sampling (SaO2).
OXYGENATION: MONITORING CONSIDERATIONS
Virtually all guidelines published to date require that patients sedated to any level must have their oxygenation monitored continuously by pulse oximetry. Observation of skin or mucosal color is helpful, it but may not signal hypoxemia until oxygen tension is critically low. Cyanosis is not detectable in most patients until hemoglobin saturations are well below 80%, which indicates that PaO2 is <50 mm Hg.
The pulse oximeter comprises a light-emitting diode that measures the absorption of specific wavelengths of light that differ between oxygenated and deoxygenated hemoglobin. Light at a wavelength of 660 nm (red) is selectively absorbed by oxygenated hemoglobin, and light at a wavelength of 940 nm (infrared) is absorbed by deoxygenated hemoglobin. The ratio of light absorptions is calculated by the pulse oximeter according to an internal computer algorithm to give a reading of a patient’s arterial hemoglobin oxygen saturation (SpO2). Readings are dependent on pulsatile blood flow because measurements are taken at the point of maximum intensity of the waveform. This allows the monitor to also compute and display pulse rate.
The probe is normally placed on a finger or toe, but sensors are also available for the nose, ear, and cheek. Regardless of placement, readings can be altered by physiologic and technical factors. The pulse oximeter is dependent on pulsatile blood flow. Anything that would cause a low flow state in the peripheral vascular beds, such as hypotension, hypothermia, or vasoconstriction, will interfere with accurate measurement. Ambient light can interfere with the wavelengths detected by the pulse oximeter, and patients who are awake, agitated, or shivering can cause motion artifact and probe displacement. Dark or metallic nail polish and acrylic fingernails can also produce spurious readings.
It must be reemphasized that pulse oximetry measures percentage of hemoglobin saturation, not PaO2. This reading must be extrapolated from the SpO2 reading displayed on the monitor screen. A useful caveat is to memorize the significance of two saturation readings; 95% and 90%. An SpO2 of 95 reflects a PaO2 of ~80 mm Hg. This is by definition the lower limit of normal oxygenation. The precise oxygen tensions for saturations above 95 are irrelevant; they reflect normal PaO2. An SpO2 of 90 reflects a PaO2 of ~60 mm Hg. By definition, this is hypoxemia, but it is well above the normal intracellular oxygen tension of 40 mm Hg. It is the most conventional alarm setting among practicing anesthesiologists. More importantly, 90% saturation represents a critical point on the oxygen-hemoglobin dissociation curve (Figure 1). Further decline leads to a precipitous drop in saturation, thus oxygenation, as hemoglobin loses oxygen rapidly. Saturation that declines to 90% is at the ‘‘edge of the cliff’’ and is a warning to aggressively reestablish adequate ventilation. PaO2 extrapolations no longer require memorization because they are ~30 less than the SpO2 reading. For example, if hemoglobin saturation is 83%, the PaO2 is ~53 mm Hg.
Finally, consider the fact that information displayed by a pulse oximeter is ‘‘old news.’’ For most monitors, detection and calculation take approximately 30 to 40 seconds before they are displayed. A monitor that alarms and displays saturations dropping into the 80s is information that occurred at least half a minute earlier; probably the result of a transient obstruction or breath holding during an injection. Conversely, once adequate ventilation is restored, it may take up to 30 seconds before pulse oximetry readings improve. This delay is a striking reconfirmation that pulse oximetry does not measure ventilation!
VENTILATION: PHYSIOLOGICAL CONSIDERATIONS
Ventilation is the movement of gases between the atmosphere and the alveoli. This must be distinguished from oxygenation. While ventilation might be normal, oxygenation can be inadequate if either the gas inhaled is lacking in oxygen or perfusion to the pulmonary alveoli is compromised. Ventilation consists of two phases: inspiration and expiration. Inspiration delivers oxygen to the alveoli and expiration delivers carbon dioxide, the by-product of cell metabolism, to the environment. Although ventilation can be initiated voluntarily, it is largely under the control of the respiratory centers of the medulla where chemoreceptors respond to elevations in hydrogen ion concentration (pH) in the following manner: Carbon dioxide (CO2) diffuses from the blood to the cerebrospinal fluid in the brain and combines with water to form carbonic acid. The acid dissociates into bicarbonate and hydrogen ions (CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3−). Although the chemoreceptors actually respond to hydrogen ions, the mechanism is referred to as hypercapnic drive because it is activated as serum carbon dioxide tensions elevate. The respiratory center can also be stimulated by neural impulses generated in the peripheral chemoreceptors of the aortic and carotid bodies. These receptors respond primarily to a decline in PaO2, and they are referred to as hypoxemic drive. Normally this mechanism assumes a secondary role to central hypercapnic drive, but assumes greater significance when central receptors become tolerant to elevated CO2 levels that occur with disorders such as chronic obstructive lung disease.
Respiratory depression refers to a reduced activity of the respiratory center in stimulating ventilation. It is a side effect of all CNS depressants, including sedatives, opioids, and general anesthetics. However, each has a predilection for depressing either hypercapnic or hypoxemic drives.5,6 Opioids primarily depress the central chemosensitive area, that is, hypercapnic drive, while inhalation anesthetics and benzodiazepines exert greater influence on the chemoreceptors in the carotid and aortic bodies, that is, hypoxemic drive. At high doses, all classes can depress both these mechanisms.
Like oxygen, the smallest portion of carbon dioxide in blood is in the free state. Most is transported as bicarbonate ion (70%), and 23% is bound to hemoglobin as carbaminohemoglobin. Only 7% of total carbon dioxide is dissolved in blood and produces a gas tension, designated PaCO2. Normal PaCO2 is approximately 40 mm Hg, and it can be measured in arterial blood gas studies along with PaO2, as described above.
While PaO2 is used to assess oxygenation, PaCO2 is the true measure of ventilation. As stated above, ventilation may be normal but the patient can be hypoxemic if the gas inhaled is deficient in oxygen or pulmonary perfusion is compromised. Conversely, a patient who is hypoventilating may be well oxygenated if he or she is breathing a gas mixture enriched with oxygen. However, PaCO2 will invariably elevate if ventilation is inadequate because carbon dioxide is not being eliminated. To summarize, a low PaO2 indicates poor oxygenation (hypoxemia) while an elevated PaCO2 (hypercarbia) indicates hypoventilation.
An incredible volume of gas occupies the extensive labyrinth of tubes and passages that compose the respiratory tract. The total lung capacity in an average young man is approximately 6000 mL, which is subcategorized into volumes and capacities, based on the proportion mobilized during various degrees of ventilatory effort. These are illustrated in Figure 2, but tidal volume and functional residual capacity deserve particular attention.
The volume of gas inspired and expired during a normal ventilatory cycle is called tidal volume and averages 500 mL or 6 mL/kg to 8 mL/kg of ideal body weight. This is far less than the total amount of gas normally found in the respiratory passages. Functional residual capacity reflects the volume of gas remaining in the lungs at the end of tidal respiration and approximates 2400 mL. The oxygen content of the gas mixture in the functional residual capacity can be viewed as oxygen reserve. If ventilation ceases, oxygen in this reserve continues to diffuse into the pulmonary capillaries. Therefore, the time from onset of apnea until hypoxemia ensues is a function of the oxygen concentration found in the functional residual capacity. This is the basis for preoxygenating a patient prior to the induction of general anesthesia, neuromuscular blockade, or tracheal intubation, as well as oxygen supplementation during sedation and general anesthesia. By increasing the oxygen content of the functional residual capacity, more time will be available for appropriate intervention if hypoventilation or apnea occurs. This time varies because functional residual capacity differs according to the age, size, and medical status of the patient7 (see Figure 3).
VENTILATION: MONITORING CONSIDERATIONS
Hypoventilation is the most significant complication attributable to sedation and general anesthesia. It is crucial that one appreciate that hypoventilation can result from not only respiratory depression, but airway obstruction. In fact, the benzodiazepines and conventional doses of opioids used for most moderate sedation techniques produce only mild degrees of respiratory depression. Far more significant is that the relaxed patient is more likely to develop anatomical airway obstruction from soft tissues (eg, tongue, tonsils, or adenoids).
The ability of patients to maintain airway patency declines as they become more sedated. As depth of sedation increases, upper airway muscle tone becomes more relaxed. The tongue falls backward and, along with relaxation of pharyngeal musculature, the airway becomes compromised (anatomical airway obstruction). These events are potentiated by the patient’s recumbent position and the nature of intraoral dental procedures. Without a patent airway, ventilation is impaired despite an intact respiratory drive. Patients who are moderately sedated generally maintain their airway without assistance, unless they are obese or they suffer from medical conditions such as obstructive sleep apnea. In contrast, patients intentionally or unintentionally sedated more deeply almost always require some assistance to maintain a patent airway. If one is to assure adequate ventilation, airway patency must be sustained. This objective is the most essential caveat for the safe use of sedation and anesthesia.
Monitoring ventilation can be difficult. During mild and moderate sedation using nitrous oxide, deflation and subsequent inflation of the reservoir bag is useful in assessing ventilation. Observation of chest movement and auditory clues are also helpful. An increase in sonorous breath sounds may be a sign of increasing airway obstruction. Nasal flaring, discordant chest wall motion, and retraction at the suprasternal area (tracheal tug) with respiratory effort are all physical signs of airway obstruction. During inspiration the chest wall normally expands upward and outward but with airway obstruction, the chest wall appears to ‘‘rock.’’ The patient’s attempts at ventilation causes the chest wall to contract inward and the abdomen to push outward. Similarly, the area of the trachea above the sternal notch will retract with respiratory effort. The most effective way to manage anatomical obstruction is to extend the head and lift the chin. If this fails to establish patency, the mandible should be displaced anteriorly, completing the so-called triple airway maneuver (head tilt-chin lift-jaw thrust).
A weighted precordial stethoscope can be used to monitor airway patency and ventilation. This device is similar to the conventional bell-shaped stethoscope but heavier so as to maintain its position over the suprasternal notch. Double-sided adhesive disks can be used to further stabilize its position and provide an acoustic seal against the patient’s skin. Electronic devices are available that amplify the sounds so they are more easily heard, for example, Bluetooth devices (see Figure 4). Precordial auscultation is exceptional for continuous monitoring of breath sounds and air exchange.
Some ECG monitoring systems record respiratory rate by impedance plethysmography. In addition to recording cardiac activity, the ECG electrodes sense chest-wall motion, and respiratory rate is displayed. However, this system fails to detect airway obstruction because it cannot distinguish normal from abnormal wall movements. It cannot be considered an adequate monitor of ventilation.
The purest measure of adequate ventilation is by assessing carbon dioxide tension. Obviously, continuous sampling of arterial blood gases is impractical. However, the tension of carbon dioxide in expired gas, particularly at the end of a tidal expiration, closely approximates the tension in arterial blood. While normal PaCO2 is ~40 mm Hg, so-called end-tidal CO2 (ETCO2) is ~35 mm Hg to 38 mm Hg. This measurement is the basis of capnometry.
Capnometry is the measurement of carbon dioxide concentration during the respiratory cycle. It uses infrared technology to analyze carbon dioxide in exhaled gas. There are multiple options to facilitate sampling of carbon dioxide. The most accurate readings are those obtained by sampling gases in the endotracheal tube of an intubated patient. However, during moderate and deep sedation the patient is not intubated, so other devices have been developed for gas sampling. Special nasal cannulas, designed to provide supplemental oxygen during sedation, also have a sampling line included. Cannulas without this feature can be modified by placing an IV catheter through one of the nasal prongs and attaching the monitor sampling line to the catheter hub.
Capnography is the proper term for those monitors that display a continuous waveform reflecting inspiration and expiration. While capnometers and capnographs both display numeric values for ETCO2 and respiratory rate, capnography is preferred because visualization of the waveform allows continuous assessment of the depth and frequency of each ventilatory cycle. Respiratory depression produces a reduction in number of waveforms while obstructions alter the shape and height of each waveform. A typical ETCO2 waveform is illustrated in Figure 5.
SUPPLEMENTAL OXYGEN AND MONITORING
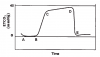
Oxygenation and ventilation are distinct physiological events. That being said, monitoring oxygenation via pulse oximetry certainly reflects ventilation in the setting of dental procedures using moderate sedation. There is little reason for oxygenation to diminish other than for reasons of hypoventilation, be it from respiratory depression or airway obstruction. However, it is only when patients breathe room air that abnormalities in ventilation can be detected reliably. The use of pulse oximetry for this purpose is impaired when using supplemental oxygen because oxygenation might remain adequate for considerable time despite the presence of hypoventilation. However, a patient breathing room air will begin to desaturate in concert with hypoventilation8 (Figure 6).
The potential benefit of supplemental oxygen must be weighed against the false sense of security conveyed to the clinician who does not monitor ventilation continuously and only monitors oxygenation by pulse oximetry. This is acceptable practice because guidelines for monitoring during moderate sedation require that ventilation be monitored only periodically (continually, not continuously). In fact, the routine administration of supplemental oxygen is probably unwarranted during moderate sedation if the patient is able to maintain adequate oxygenation in room air.9 Deitch et al10 reported a controlled trial in which there was no apparent advantage to using supplemental oxygen during moderate sedation for emergency room procedures. However, as explained earlier, supplemental oxygen increases oxygen concentration in the functional residual capacity and will delay the onset of hypoxemia should the patient experience airway obstruction, hypoventilation, or apnea. Supplementing the patient’s air with oxygen will provide additional working time to correct the problem. This, of course, presumes that ventilation is being monitored and the dentist is not relying on pulse oximetry alone. When the decision is made to provide supplemental oxygen, it is essential that ventilation be monitored either by capnography or by auscultation of breath sounds, using a precordial or pretracheal stethoscope.
There are exceptions for which supplemental oxygen should always be provided, even for mild and moderate techniques. Pediatric sedation is most notable. Children are at greater risk for airway obstruction and are more apt to experience transient episodes of apnea. Even more importantly, their functional residual capacity is much less than an adult’s and their oxygen demand is almost double that of an adult on a kilogram-for-kilogram basis.11 For these reasons, children should receive oxygen supplementation for all levels of sedation. The same can be said for obese or pregnant patients.
The key principle is that when supplemental oxygen is provided for any reason, the pulse oximeter must never be misconstrued as monitoring ventilation. During deep sedation and general anesthesia, the risk of significant respiratory depression, airway obstruction, and transient episodes of apnea mandates oxygen supplementation. For these patients, ventilation must be monitored continuously using either a precordial stethoscope or capnography.
About the Authors
Dr. Becker is an Associate Director of Education, General Dental Practice Residency, at Miami Valley Hospital in Dayton, Ohio. Dr. Casabianca is an Assistant Professor in the Department of Anesthesiology and Surgery (Dentistry) at the University of Toledo College of Medicine, in Toledo, Ohio.
REFERENCES
1. Eichhorn JH, Cooper JB, Cullen DJ, et al. Standards for patient monitoring during anesthesia at Harvard Medical School. JAMA. 1986;256:1017-1020.
2. Rosenberg MB, Campbell RL. Guidelines for intraoperative monitoring of dental patients undergoing conscious sedation, deep sedation, and general anesthesia. Oral Surg Oral Med Oral Pathol. 1991;71:2-8.
3. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists, Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017.
4. Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Philadelphia: Elsevier Inc; 2006:471-522.
5. Keats AS. The effects of drugs on respiration in man. Ann Rev Pharmacol Toxicol. 1985;25:41-65.
6. Becker DE. The respiratory effects of drugs used for conscious sedation and general anesthesia. J Am Dent Assoc. 1989;119:153-156.
7. Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg IV succinylcholine. Anesthesiology. 1997;87:979^982.
8. Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552-1558.
9. Green SM. Research advances in procedural sedation and analgesia. Ann Emerg Med. 2007;49:31-36.
10. Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Ann Emerg Med. 2007;49:1-8.
11. Cravero JP, Kain ZN. Pediatric anesthesia. In: Barash PG, Cullen BF, Stoelting RK, eds. Clinical Anesthesia. 5th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2006:1205-1218.