You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
An astounding number of drug classes and formulations are available to manage infections. Fortunately, the microorganisms associated with odontogenic and periodontal infections are well characterized and a relatively small number of antimicrobial agents are required to effectively manage dental infections. With the exception of allergy, adverse effects attributed to these antibiotics are surprisingly infrequent, but most agents are implicated in producing nausea, dyspepsia, and diarrhea. This article will review essential pharmacology of appropriate drug classes and then address clinical considerations for their therapeutic and prophylactic use.
PHARMACOLOGICAL CONSIDERATIONS
Antibiotics are generally classified according to their molecular structure and their antimicrobial mechanisms. Ideally, these mechanisms of action either interrupt synthesis of structural components or alter specific metabolic functions that are unique to microbial cells. Such specificity cannot always be accomplished, but if it is, human cells can be spared cytotoxic effects.
Beta Lactam Derivatives
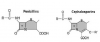
The molecular structures of penicillins and cephalosporins have a beta lactam ring in common that accounts for their ability to inhibit cell wall synthesis in susceptible microorganisms1 (Figure 1). Additional beta lactam derivatives include the monobactams and carbapenems, but these are not used for dental infections. The beta lactam ring is also the target for resistant species, which produce a variety of beta lactamases formerly called penicillinase and/or cephalosporinase. Prominent microbial species that produce beta lactamases are Staphylococcus aureus and Haemophilus influenzae. However, several species of Bacteroides and Prevotella, which contribute to the more severe odontogenic and periodontal infections, may also demonstrate this mechanism of resistance.
The similar molecular structure for penicillins and cephalosporins raises concern regarding cross-allergenicity. Formerly this was thought to be related to the beta lactam ring, but recent evidence has established that cross-allergenicity is related more to similarities in the R side chains2,3 (see Figure 1). It is generally accepted that patients having a history of IgE-mediated reaction to a penicillin should be managed using a non-beta lactam antibiotic.4 Urticaria (hives) is immunoglobulin E-mediated but accounts for only 10% of all exanthematous drug reactions. The overwhelming majority of cutaneous reactions to penicillins are pruritus or rash; these are not immunoglobulin E-mediated, and any potential for cross-reaction is unlikely.5,6 Furthermore, upon further questioning, most patients claiming allergy to penicillin are discovered to have experienced stomach upset (dyspepsia), nausea, or diarrhea. Although macrolides and clindamycin are conventionally considered the alternatives of choice in patients allergic to penicillins, the macrolides have become less attractive and will be discussed further below. It is preferable to substitute an alternate penicillin or cephalosporin for a penicillin-allergic patient, provided the nature of the patient’s reaction was merely pruritic (itch) or a maculopapular rash. A history of urticaria (hives) or anaphylactoid symptoms is more convincing evidence that the patient’s reaction to penicillin was truly immunoglobulin E-mediated, and in this case, one should refrain from prescribing any beta lactam derivative. Clinical management of the penicillin-allergic patient is summarized in Figure 2.
Penicillins. The fortuitous discovery of penicillin is credited to Alexander Fleming, and its clinical use began in 1941. This first penicillin was designated penicillin G and its dosage expressed in units or milligrams, 1600 units approximating 1 mg. It is very active against gram-positive cocci that frequently cause oral, pharyngeal, and pulmonary infections. It is also active against Neisseria gonorrhoeae and Treponema pallidum. For this reason, penicillin G is still a first-line agent for treating the two most familiar venereal diseases, syphilis and gonorrhea. Penicillin G is highly degradable in gastric acid and is generally administered only by parenteral routes, formulated as several salts that differ in their rate of absorption. Potassium and sodium salts are used for intravenous administration and procaine or benzathine salts for intramuscular injection. Benzathine formulations provide low serum levels for 3 to 4 weeks and are generally reserved for situations in which patient compliance with oral administration is unlikely.
The phenoxymethyl derivative of penicillin, designated penicillin V, is more acid stable and has become the standard penicillin for oral use. Its spectrum is similar to that for penicillin G, but it is less active against Neisseria species and several anaerobes. It remains the agent of choice for mild to moderate intraoral infections but is susceptible to a variety of beta lactamases produced by most strains of S aureus and many species of Bacteroides.7 Although the former are seldom causative agents in oral infections, resistant strains of Bacteroides represent a significant cause for penicillin failure. This includes several species of Bacteroides that have been reclassified as other genera, either Porphyromonas or Prevotella.
To counter microbial resistance attributed to beta lactamase, derivatives of penicillin have been synthesized that are not susceptible to enzymatic breakdown. These penicillinase-stable agents include oxacillin and dicloxacillin. They are primarily indicated in the management of infections attributed to S aureus, over 90% of which are resistant to penicillin G and V. These microbes are rarely if ever present in odontogenic infections, and both oxacillin and dicloxacillin are less active than penicillin V against odontogenic pathogens.
Ampicillin was the first derivative to have an extended spectrum that includes several gram-negative organisms such as H influenzae and Escherichia coli, but these are rarely, if ever, associated with intraoral infections. A possible exception may be infections that also involve the maxillary or nasal sinuses in which H influenzae is a common culprit. Amoxicillin has the identical spectrum of activity attributed to ampicillin, but it exhibits greater oral bioavailability, and for this reason ampicillin is generally reserved for parenteral use. The bioavailability of amoxicillin is also superior to that of penicillin V and accounts for its replacing penicillin V in current American Heart Association guidelines for the prophylaxis of infective endocarditis.
Amoxicillin and penicillin V are equally active against streptococci. The only advantages of amoxicillin for dental infections are greater bioavailability and a longer half-life. For this reason, if one adheres to the principle of choosing antibiotics having the narrowest spectrum possible, penicillin V is preferred over amoxicillin. Routine use of amoxicillin fosters the accumulation of amoxicillin-resistant microorganisms that lack dental implications. However, if one considers that the leading cause of therapeutic failure is lack of patient compliance, superior bioavailability and less frequent dosing (3 times daily [TID] versus 4 times daily [QID] for penicillin V) favor the use of amoxicillin.
Ampicillin and amoxicillin are not beta lactamase stable and are available in formulations that include so-called beta lactamase inhibitors, sulbactam and clavulanic acid respectively. Clavulanic acid has a molecular structure similar to that of penicillin and has weak antimicrobial action but strong affinity for beta lactamase. It is combined with amoxicillin to act as a ‘‘suicide molecule’’ protecting it from beta lactamase (see Figure 3). The combination of amoxicillin and clavulanic acid (Augmentin) is very expensive and should not be used for routine empiric therapy. Resistance among streptococci and most other dental pathogens is not attributed to beta lactamase. At best, the use of this product may be a heroic option in refractory odontogenic and periodontal infections that, on some occasions, become colonized by penicillin-resistant Bacteroides and Prevotella species that produce this enzyme.8 However, these species are anaerobic and highly susceptible to metronidazole, which is less expensive and will be addressed later in this review. A more justifiable indication for amoxicillin-clavulanic acid would be a sinus graft infection in which subspecies of H influenzae frequently produce beta lactamase.
Cephalosporins. The first generation of cephalosporins has a spectrum of activity that includes that of penicillin V for odontogenic microbes. They are also active against most strains of S aureus because, unlike penicillin, they are not susceptible to beta lactamases produced by this species. S aureus is a common nosocomial pathogen and, for this reason, the cephalosporins are ordered more frequently than the penicillins for hospitalized patients. First-generation cephalosporins offer few advantages over penicillins in the management of dental infections. As mentioned earlier, they are an alternative for the penicillin-allergic patient, and certain agents have pharmacokinetic advantages that allow less frequent dosing. For example, the long elimination half-life of cefadroxil (Duricef) allows twice daily (BID) oral dosing, whereas cephalexin (Keflex) is administered QID. Cefazolin (Ancef, Kefzol) is the standard first-generation agent for parenteral use.
Second- and third-generation cephalosporins exhibit an even broader spectrum and greater resistance to beta lactamase. Several of the third-generation agents also demonstrate antipseudomonal activity. Although these antibiotics have valuable indications in the management of specific infectious diseases, too often they are prescribed inappropriately for infections that could be managed using less expensive agents. They are rarely if ever indicated for managing oral infections.
Macrolides
Macrolide antibiotics exert their antibacterial action by binding to the 50S ribosomal subunit of susceptible organisms, resulting in inhibition of protein synthesis.1 Erythromycin is the prototypic macrolide and has been prescribed historically as an alternative for patients allergic to penicillin because it formerly had reasonable activity against most penicillin-sensitive microbes. This is no longer the case, however. Macrolides have little activity against periodontal pathogens, and in recent years their activity against streptococcal species has declined to such a degree that most experts discourage their use in odontogenic infections as well. Desimone et al9 analyzed 150 cases of infective endocarditis in Olmstead County, Minnesota, that occurred from 1999 through 2010. Of 22 cases attributable to viridans group streptococci, ~95% were sensitive to penicillin but only ~71% to macrolides. It should be noted that streptococci and staphylococci that are resistant to erythromycin are also resistant to clarithromycin and azithromycin.7 Clarithromycin and azithromycin are more active than erythromycin against Streptococcus viridans, which accounts for their mention as penicillin alternatives in current American Heart Association guidelines for prophylaxis of infective endocarditis.10 However, it will not be surprising to see this revised in future guidelines. In addition to growing resistance among odontogenic pathogens, macrolides produce a high incidence of nausea, and the majority of these agents inhibit cytochrome P450 enzymes. This accounts for a staggering number of potential drug interactions. Furthermore, azithromycin continues to be a concern for promoting cardiac arrhythmias in susceptible patients.11
Tetracyclines
Like the macrolides, tetracyclines are bacteriostatic but exert their antimicrobial effect by binding to the 30S subunit of the bacterial ribosome to inhibit protein synthesis.1 Tetracyclines have a wide spectrum of activity, but microbial resistance has increased to the extent that they are seldom first-line agents for medically treated infections. Sinus and respiratory infections caused by H influenzae and pneumonococci are notable exceptions because most of these strains remain sensitive.
In dental practice, tetracyclines are useful adjuncts for managing periodontal infections. They are highly active against many of the microorganisms implicated in gingival and periodontal disease and exhibit high bioavailability in the gingival sulcus. However, their efficacy is unreliable for managing odontogenic infections due to streptococcal resistance. Notably, there is less antimicrobial resistance to doxycycline and minocycline than to other, more conventional tetracyclines, and these are generally preferred for periodontal infection.
Like other tetracyclines, doxycycline (Vibramycin) increases skin sensitivity to sunlight, leading to intense sunburn and generalized erythema, but offers several advantages. It is well absorbed in the presence of food and has an extended elimination half-life that allows for once-daily dosing. It is eliminated primarily in feces, and this makes it particularly attractive for patients having hepatic or renal compromise. Finally, even at subantimicrobial doses, doxycycline inhibits the activity of collagenases that contribute to the pathogenesis of periodontal destruction.
Metronidazole
Metronidazole is a prodrug that is converted to a toxic radical within anaerobic microbes. The radical destroys existing DNA and other vital compounds, rendering it bactericidal against most anaerobic organisms.1 For this reason, it is very useful for treating severe odontogenic and periodontal infections where anaerobes are able to thrive. It is not recommended as monotherapy for oral infections because it is inactive against aerobic and facultative streptococci. However, it may be combined with beta lactams when managing severe refractory infections. Patients should be cautioned to avoid alcoholic beverages while taking this medication because disulfiram-like reactions have been reported. These consist of severe nausea and abdominal cramping due to the formation of a toxic compound resembling formaldehyde.
Clindamycin
Clindamycin binds to the 50S subunit of bacterial ribosomes to suppress protein synthesis, but, unlike the macrolides, it is bacteriocidal.1 It has reliable activity against both aerobic and anaerobic cocci, as well as most species of Bacteroides, including Bacteroides fragilis. These pathogens are often implicated in severe orofacial infections. Its cost and predilection for Clostridium difficile infection limit its routine use for dental infections in favor of beta lactams. However, these should not be deterrents to using clindamycin when indicated. Indeed, C difficile infection may be a complication associated with amoxicillin and cephalosporins as well.
Antifungal Agents
Nystatin was formerly the most common antifungal agent used for oral candida infections but today the azole derivatives are preferred. These agents inhibit the synthesis of ergosterol, an essential component of the fungal cell membrane. Although a variety of agents are available, clotrimazole (Mycelex) troches are generally preferred based on cost and little risk for side effects and drug interactions. Clotrimazole can be prescribed as 10-mg troches administered 5 times daily for 10 to 14 days.12 Fluconazole (Diflucan) is available for oral (PO) administration and miconazole (Oravig) as once-daily buccal tablets, but they are very expensive. These 2 azoles also inhibit several families of cytochrome P450 enzymes and should be avoided in patients taking warfarin, statins, antiretrovirals, and any drug known to prolong QT intervals.12,13
Miscellaneous Agents
The following agents have little or no indications for managing odontogenic or periodontal infections. However, some familiarity is essential for collaborative medical management of dental patients. The most significant risk for infective endocarditis is in patients having a prior history of this infection, especially those with prosthetic valves. In these cases, some cardiologists may prefer that an aminoglycoside such as gentamicin be added to the prophylactic regimen. This is based on the synergistic influence these agents have with cell wall inhibitors in killing enterococci and resistant strains of S viridans. However, there is little scientific evidence to support this practice.14 For high-risk patients who are allergic to beta lactams, vancomycin may be requested and does not require the addition of gentamicin. These antibiotics must be administered by intravenous infusion over 30 to 60 minutes preoperatively, which may require that treatment be arranged at an outpatient clinic if the provider is inexperienced with this route of administration.
Aminoglycosides. Historically, the most familiar member of this class is streptomycin, formerly the agent of choice for treating tuberculosis. Gentamicin and tobramycin are used most commonly and are the primary agents used to treat infections caused by gram-negative rods, most notably Pseudomonas species. Although most antibiotics that inhibit protein synthesis are bacteriostatic, the aminoglycosides are frequently bactericidal. The newer generations of beta lactam antibiotics are also active against Pseudomonas species, but they are far more expensive.
The major drawback of the aminoglycosides is their toxicity. This includes nephrotoxicity and ototoxicity. Nephrotoxicity is fairly common, but is generally reversible following discontinuation of the offending drug. Ototoxicity may include either the auditory or vestibular portions of the eighth cranial nerve. Unlike renal cells, sensory neurons cannot regenerate, and toxicity may be permanent. Hearing and labyrinthine function must be carefully monitored because initial symptoms may be reversible provided the drug is withdrawn quickly. Tinnitus is the earliest sign of auditory toxicity, whereas headache and nausea generally herald the onset of vestibular toxicity. The various preparations differ in their propensity to induce toxicity in each division.
Vancomycin. Vancomycin inhibits cell wall synthesis and is active against most gram-positive cocci, including most species of streptococci, staphylococci, and enterococci.1 Although enterococci were once uniformly susceptible to vancomycin, outbreaks of infections caused by resistant strains are a growing problem. Scattered cases of infections caused by vancomycin-resistant enterococci are particularly sobering because they frequently result in mortality. Consequently, vancomycin is reserved for treatment of serious infections caused by organisms that are resistant to first-line agents, such as beta lactams, or in cases of serious allergy to these antibiotics. This may include patients at great risk for infective endocarditis who are allergic to beta lactams, as an alternative to clindamycin.
Vancomycin can produce pseudoallergic reactions. Rapid intravenous infusion may trigger histamine release, which causes a variety of symptoms, including erythematous or urticarial reactions, flushing, tachycardia, and hypotension. The extreme flushing is called redman syndrome. The most significant untoward reactions are ototoxicity and nephrotoxicity. Auditory impairment can be permanent, especially with excessively high plasma concentrations. Nephrotoxicity is less common, but renal function should be monitored and appropriate plasma concentrations maintained. As might be expected, toxicity is more likely when administered concurrently with an aminoglycoside.
Fluoroquinolones. The fluoroquinolones are synthetic, broad-spectrum antibacterial agents that inhibit DNA gyrase, an essential enzyme that is involved in the replication, transcription, and repair of bacterial DNA.1 The introduction of quinolone derivatives is the most significant recent advance in antimicrobial therapy. Ciprofloxacin (Cipro) was the first of these agents introduced and is regarded as the prototype. It is active against most staphylococci and a variety of gram-negative microorganisms, but has poor activity against most streptococci and all anaerobes. This negates its use for odontogenic and periodontal infections, which generally consist of mixed aerobic and anaerobic flora (see Table 1). Newer generations of quinolones have broader activity, but their cost generally renders them inappropriate for dental-related infections. Levofloxacin (Levaquin) has good antistreptococci action but poor anaerobic activity. Once daily dosing is attractive, but its cost is ~$25/d and its extensive antimicrobial spectrumcompletely overrules conventional principles of antibiotic prescribing for odontogenic infections. Gemifloxacin (Factive) offers added anaerobic coverage but at a cost of ~$50/d. At best it might be argued as an outrageously expensive effort to heroically salvage a persistent peri-implant infection.
ANTIBIOTIC COMPLICATIONS
There are surprisingly few complications associated with antibiotic therapy. Other than allergy to beta lactam agents addressed previously, the principal complications are gastrointestinal related and potential drug interactions.
Antibiotic-Associated Diarrhea and Colitis
Antibiotic-associated diarrhea is not that uncommon during a course of antibiotic therapy, but it becomes a more significant event if it is the result of C difficile infection, a common nosocomial pathogen. This anaerobic, spore-forming bacillus is most often contracted during hospitalization or prolonged stays in other healthcare facilities. However, it is also found in the community, and this source of infection is on the rise. Intestinal flora normally prevent colonization by C difficile, and it is present in only 1% to 4% of the general population, but 0.20% in those admitted to health care facilities for a week or more.15,16 When normal flora is altered by antibiotic therapy and the patient either harbors or comes into contact with C difficile, colonization increases. Colonization may be enhanced by most antibiotics, but clindamycin, amoxicillin, second- and third-generation cephalosporins, and the fluoroquinolones are most often implicated.15
Colonization alone does not necessarily result in C difficile infection. Risk for actual infection depends on the interaction of several additional factors, including virulence of the particular strain and patient-related factors such as age, immune status, and the concurrent use of acid-reduction gastrointestinal drugs, eg, proton pump inhibitors. Once C difficile infection occurs, the consequences range from diarrhea to pseudomembranous colitis. Therefore, in outpatient dental practice the typical sequence of events leading to C difficile infection are as follows:
1. The patient is currently colonized with C difficile. (This is most likely if the patient has recently visited, has been a patient, or is a healthcare provider in a hospital or nursing home.)
2. Colonization is then increased by an antibiotic altering intestinal flora. (Clindamycin or amoxicillin are most likely.)
3. Patient-related factors determine risk for actual infection and subsequent severity. (Many variables contribute, but older age, poor immune status, and use of acid-reduction drugs are most significant.)
The incidence of diarrhea attributed to those antibiotics commonly used in dentistry ranges from 2% to 10% and may be as high as 25% with amoxicillin/clavulanic acid (Augmentin).17 Clinically, the challenge is to distinguish this so-called nuisance diarrhea from that associated with C difficile disease. In a patient who normally tolerates antibiotics but experiences diarrhea that is florid (>3 unformed stools per day for >2 days) and complains of abdominal pain, C difficile infection should be suspected. Milder symptoms in patients who have previously experienced diarrhea with antibiotics favor nuisance diarrhea.15,17
Mild, nuisance diarrhea may be managed using antiperistaltics and changing the antibiotic to a narrower spectrum if possible. However, if diarrhea is severe and C difficile infection is suspected, the following is suggested:15-18
1. Avoid antiperistaltics. Accumulation of toxin can worsen the infection.
2. Stop the current antibiotic and prescribe metronidazole 500 mg TID 3 10 to 14 days.
3. If there is no improvement after 2 to 3 days (based on severity), or diarrhea subsides and recurs, refer the patient to his or her family physician, who will evaluate fluid/electrolyte status. For severe cases, the physician may switch metronidazole to oral vancomycin, which is not absorbed but provides its action locally within the colon. However, oral vancomycin is shockingly expensive and will be initiated only in extreme cases.
It is significant that C difficile infection has been reported to occur as late as 6 to 8 weeks following clindamycin use and has also been associated with the use of macrolides, tetracycline, and most of the broader-spectrum beta lactam antibiotics. This complication is unheard of following abbreviated use of clindamycin for prophylaxis of infective endocarditis.
The use of probiotics to prevent or manage antibiotic-associated diarrhea remains controversial. Nevertheless, current evidence suggests they are indeed effective and should be suggested for particularly frail patients or those who have experienced diarrhea with antibiotic regimens in the past.19
Antibiotic Drug Interactions
Like all drug classes, antibiotics pose a risk for drug interactions. Since the early 1980s, numerous articles have suggested that antibiotics may reduce the efficacy of oral contraceptives (OCs). However, most of these publications have been either anecdotal reports or elaborate theories based on these reports. Unfortunately, many of these are found in dental literature. Anonymous cases are frequently cited in these careless reports and mention dentists who were sued for child support following an unwanted pregnancy attributed to antibiotic-OC interaction. None of these case reports cite legal proceedings that can be researched, and these reports should be viewed with suspicion. To date, rifampin (an antituberculosis agent) is the only antibiotic having a confirmed interaction with OCs.20 Despite the equivocal status of this issue, the Physicians’ Desk Reference and other drug compendia continue to mention the possibility of this interaction.
In a thorough review of OCs, Sondheimer21 stated that the generally accepted rate of unwanted pregnancy among OC users is 0.5 to 1% (8% among teens) and the most common reason for failure is noncompliance. Furthermore, he added that most antibiotics do not decrease the effectiveness of the birth control pill. It is entirely within reason to suspect that a woman might also be taking an antibiotic during the month of OC failure, most likely penicillin or tetracycline. To date, all human studies measuring the influence of antibiotics on estrogen and progestin serum concentrations have found no interaction with antibiotics other than rifampin.1,22-24 There is simply no sound evidence to support the contention that antibiotics other than rifampin reduce the effectiveness of OCs.
Fortunately, the beta lactam antibiotics used to manage dental infections are virtually free of any significant drug interactions. The macrolides are an entirely different matter, however. They not only have an inherent potential to lengthen cardiac repolarization and prolong QT intervals but inhibit the metabolism of many drugs having even more potential for this cardiotoxic effect.11,25 They also delay metabolism and elevate serum concentrations of additional drugs, leading to potential toxicity. These include warfarin, digoxin, carbamazepine, valproic acid, ergotamine, and theophyllines. This alarming list of potential drug interactions, combined with their declining activity against dental pathogens, renders the routine use of macrolides questionable.
The potential for interactions with warfarin deserves particular mention. Along with macrolides, metronidazole and azole antifungals delay the metabolism of warfarin and must always be avoided. Other antibiotics have some potential for enhancing warfarin effects by reducing bowel flora that produce vitamin K, but this potential is generally not a concern. Nonetheless, caution is advised when caring for older patients. A recent publication found that exposure to several classes of antimicrobials, particularly azole antifungals, was associated with an increased risk of bleeding in this population receiving warfarin.26
Finally, doxycycline may elevate digoxin levels and should be avoided. It has also been found to enhance the hypoglycemic influences of exogenously administered insulin. Diabetics receiving insulin should be cautioned to monitor their glucose closely if doxycycline is prescribed.
CLINICAL CONSIDERATIONS
For good reason, the selection of a particular antibiotic is based on empirical judgment rather than culture and sensitivity data. This is because the most common pathogens implicated in oral infections have been well defined and the most effective antibiotics confirmed.7,8,27
The effectiveness of a particular antibiotic for managing specific infections is predicated on three variables: (a) the antibiotic’s minimum inhibitory concentration (MIC)-90 for the pathogen, (b) its peak serum level, and (c) its elimination half-life.
a. The MIC-90 represents the serum concentration required to inhibit or destroy 90% of the species for a selected class of microorganisms. It is important to consider that an antibiotic may exhibit activity in vitro, but it is of little use if this concentration cannot be achieved in the tissue infected.
b. Following PO administration, serum concentration must exceed the MIC-90.
c. An antibiotic is ineffective if its elimination pattern does not sustain an acceptable serum concentration for a reasonable period of time. Concentration drops by 50% each half-life.
Data for the application of these principles to antibiotics used in dental practice are presented in Table 1. Precise data will vary depending on the reference text consulted, but will be reasonably similar to that derived from Mandell et al.7
Treatment of Existing Infection
There are three essential principles to follow when managing odontogenic and periodontal infections:
1. The source of the infection should be removed.
2. If the infection is too severe for the patient’s natural immune system to manage, antibiotic therapy is indicated.
3. Select an antibiotic with the narrowest spectrum that includes the most likely pathogens.
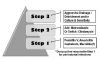
A stepped approach can be used to empirically select antibiotics for managing existing dental infections. Treatment should commence with either a penicillin, a cephalosporin, or, rarely, a macrolide. Antibiotic therapy does not obviate the need for surgical drainage when this is possible. If the condition deteriorates or fails to improve over the subsequent 2 to 3 days, either add metronidazole to the current regimen or switch the regimen to clindamycin. This principle is illustrated in Figure 4. If this second step fails, more aggressive surgical drainage is indicated and culture/sensitivity data may be used to guide further antibiotic selection. If cellulitis evolves, a dentist is wise to at least consult, and preferably to refer the patient to, an oral and maxillofacial surgeon for definitive management.
The duration of antibiotic coverage is empiric; there are no precise standards for duration of coverage. Most dental-related infections will resolve within 5 to 7 days and, provided the patient’s signs and symptoms resolve, there is little reason to provide longer coverage. The idea that coverage should extend for several days following complete remission to prevent recurrence is a misconception. Dental infections do not rebound provided the source of infection has been corrected.
The dose of the antibiotic selected should achieve levels in the infected tissues that exceed the MIC-90 for the targeted microorganisms. The actual concentrations attained in various tissues are not always as high as those in serum, so it is prudent to strive for persistent serum levels at least 2 to 5 times the MIC-90. For severe infections it may be wise to double the first dose (loading dose) to establish a high initial concentration to promote distribution into the infected tissues. Antibiotic dosages for managing dental infections are summarized in Table 2.
The elimination half-life of the antibiotic will predict the rate of decline in the serum level, and this consideration is used by the drug manufacturer in establishing the recommended dosing interval. Provided the patient is compliant with the published dosing schedule, serum levels will remain above the MIC-90. Compliance is most critical for the beta lactam antibiotics because of their mechanism of action. These drugs interfere with cell wall synthesis and must be present in adequate concentration at any time the microbe attempts cell division. Other classes of antibiotics, such as macrolides, clindamycin, tetracycline, and metronidazole, are somewhat forgiving because they exhibit so-called post-antibiotic effects. This property defines the antibiotic’s ability to continue its influence on microorganisms for varying periods of time after the serum level has declined below the MIC-90. There are many mechanisms proposed for the post-antibiotic effects of various antibiotics, including an ability to reside within the cytoplasm of the microbes, suppressing their biochemical pathways (metronidazole), or residing within macrophages, where microbes become exposed during phagocytosis (macrolides).
Routine Surgical Prophylaxis
The concept of surgical prophylaxis is often misunderstood. Its premise is that the surgical site is currently not infected, but may become contaminated during surgery. This condition is not present when removing abscessed or periodontally compromised teeth; infection is currently present. Prescribing antibiotics during or following these cases is not prophylaxis. It represents therapeutic use of antibiotics to manage infected tissues and is frequently unnecessary after the offending culprits are surgically removed.
Antibiotic prophylaxis may be indicated when opening flaps to remove impactions or for dental implants and grafting procedures. In these cases, the surgical site is not infected at the start of surgery, and it may be appropriate to administer an antibiotic preoperatively to decrease the likelihood of introducing an infection. When using antibiotic prophylaxis, an adequate serum concentration should be established no earlier than 2 hours before a surgical incision is made, and should not be repeated unless the procedure is prolonged (0.3 hours). In this case a dose may be repeated at intervals corresponding to 1 to 2 elimination half-lives for the drug used.28 Prolonged coverage will encourage growth of resistant organisms, and this may be especially relevant for success with implant placements. There is absolutely no rationale to commence antibiotic therapy following surgery, deeming it prophylaxis.
A reasonable prophylaxis regimen for dental surgery would be the amoxicillin regimen suggested for prevention of infective endocarditis. For those preferring an intravenous regimen, cefazolin (Ancef, Kefzol) is attractive because it is inexpensive and provides effective coverage for 8 hours, similar to amoxicillin. This can be followed with an oral cephalosporin or amoxicillin to extend the coverage, but additional doses are generally not recommended, certainly for no more than 24 hours.28 Suggested regimens for routine surgical prophylaxis are summarized in Table 3.
Medical Prophylaxis
Unlike routine surgical prophylaxis, medical indications for antibiotic prophylaxis are predicated neither on the presence nor on the absence of current infection. The basis for these indications is either a great potential for postoperative infection of the surgical site or distant-site infections attributable to surgically induced bacteremia.
Immunocompromised patients heal poorly and are at greater risk for developing infections. Examples of conditions that may be associated with poor immune status include the following, and dentists often provide a course of antibiotics when performing dental surgery for these patients:
• Poorly controlled diabetes.
• Systemic lupus erythematosus.
• End-stage renal disease undergoing dialysis.
• Evidence of significant malnutrition or alcoholism.
• Symptomatic human immunodeficiency virus-positive patients.
• Patients receiving immunosuppressant drugs or radiation to head and neck.
Although some authors suggest this practice, there are no published guidelines. Lockhart et al29 have published an impressive review of scientific literature and found no scientific basis for this practice. Nevertheless, the dentist may empirically justify coverage in some cases. If prophylaxis is elected, however, it is doubtful that preoperative doses provide any particular benefit; coverage may commence postoperatively and continue for 5 to 7 days. The only exception is the dialysis patient with an arteriovenous shunt. For these patients, preoperative prophylaxis may be indicated, but only if performing incision and drainage of an abscess.29
Other medical indications for antibiotic prophylaxis are predicated on a potential for distant-site infections as a consequences of bacteremia, but even these are frequently challenged. Surgical and invasive diagnostic procedures may certainly introduce bacteria into the bloodstream (bacteremia), but this is of little consequence unless a patient has some distant site that is susceptible to microbial colonization. Unfortunately, there is little science to establish which sites or devices are actually susceptible to colonization.
The American Heart Association recommends that patients at risk for infective endocarditis receive prophylactic antibiotics prior to dental and other surgical procedures. The antibiotics recommended are those that are most active against the specific microbes likely to be introduced during the specific procedure. The most recent guidelines for prevention of infective endocarditis in patients undergoing dental procedures were published in 2007 and are summarized in Table 4.10 It is significant that former concerns regarding valvular disease, eg, murmurs, are no longer recommended for prophylaxis. Although penicillins are generally preferred, macrolides, clindamycin, and cephalosporins are acceptable alternatives for patients having a penicillin allergy. As mentioned previously, resistance to macrolides is increasing and any recommendation for their use may be modified in future guidelines.9
In some cases, a patient may present for treatment having not premedicated. The following is a direct quote from the current American Heart Association guidelines; simply stated, administer the antibiotic regimen and complete the treatment!
"An antibiotic for prophylaxis should be administered in a single dose before the procedure. If the dosage of antibiotic is inadvertently not administered before the procedure, the dosage may be administered up to 2 hours after the procedure. However, administration of the dosage after the procedure should be considered only when the patient did not receive the pre-procedure dose."10
The risk for hematogenous seeding and infection of total joint prostheses are more controversial. Little information has been published on this matter, but Berbari et al30 have published the most significant research and found no increased risk. The American Dental Association and American Academy of Orthopaedic Surgeons published updated guidelines in 2012 that suggest discontinuing routine antibiotic prophylaxis for all patients having joint replacements.31 A summary of these rather vague guidelines is as follows:
1. The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.
2. We are unable to recommend for or against the use of topical oral antimicrobials in patients with prosthetic joint implants or other orthopaedic implants undergoing dental procedures.
3. In the absence of reliable evidence linking poor oral health to prosthetic joint infection, it is the opinion of the work group that patients with prosthetic joint implants or other orthopaedic implants maintain appropriate oral hygiene.
Regardless of continued controversy, the dentist may still encounter a patient whose orthopedist requests antibiotic coverage. In this case it is my personal practice to honor the request and cover the patient using regimens identical to those suggested for prophylaxis of infective endocarditis. Unless a patient volunteers his or her orthopedist’s request for antibiotic prophylaxis, I do not broach the subject.
About the Author
Dr. Becker is an Associate Director of Education, General Dental Practice Residency, at Miami Valley Hospital in Dayton, Ohio.
REFERENCES
1. Drug Facts and Comparisons in Clin-Eguide. New York, NY: Wolters Kluwer Health; 2012.
2. Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med. 2012;42:612–620.
3. Solensky R. Allergy to beta-lactam antibiotics. J Allergy Clin Immunol. 2012;130:1442.
4. Abramowicz M, ed. Cephalosporins for patients with penicillin allergy. Med Lett Drugs Ther. 2012;54:101.
5. Stern RS. Clinical practice. Exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
6. Solensky R, Kahn DA. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
7. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Churchill Livingston (Elsevier), 2009.
8. Kuriyama T, Karasawa T, Nakagawa K, et al. Incidence of beta-lactamase production and antimicrobial susceptibility of anaerobic gram-negative rods isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol Immunol. 2001;16:10-15.
9. Desimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association’s endocarditis prevention guidelines. Circulation. 2012;126:60-64.
10. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736-1754.
11. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
12. Abramowicz M, ed. Miconazole (Oravig) for oropharyngeal candidiasis. Med Lett. 2010;52:95-96.
13. Abramowicz M, ed. Antifungal drugs. Treat Guidel Med Lett. 2012;10:62-63.
14. Gumbo T. General principles of antimicrobial therapy. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill Companies Inc; 2011.
15. Gerding DN, Johnson S. Clostridium difficile-associated disease, including pseudomembranous colitis. In: Longo DL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw Hill; 2012.
16. Kelly CP. A 76-year-old man with recurrent Clostridium difficile-associated diarrhea: review of C. difficile infection. JAMA. 2009;301:954-962.
17. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334-339.
18. Abramowicz M, ed. Treatment of Clostridium difficile infection. Med Lett Drugs Ther. 2011;1358:14-15.
19. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
20. Reimers D, Jezek A. The simultaneous use of rifampicin and other antitubercular agents with oral contraceptives. Prax Klin Pneumol. 1971;25:255-262.
21. Sondheimer SJ. Update on oral contraceptive pills and postcoital contraception. Curr Opin Obstet Gynecol. 1992;4:502-505.
22. Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472-484.
23. Becker DE. Drug interactions in dental practice: a summary of facts and controversies. Compendium. 1994;15:1228-1242.
24. Miller DM, Helms SE, Brodell RT. A practical approach to antibiotic treatment in women taking oral contraceptives. J Am Acad Dermatol. 1994;30:1008-1011.
25. Ray WA, Murray KT, Meredith S, et al. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089-1096.
26. Baillargeon J, Holmes HM, Lin YL, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
27. Kuriyama T, Karasawa T, Nakagawa K, et al. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:600-608.
28. Abramowicz M, ed. Antimicrobial prophylaxis for surgery. Treat Guidel Med Lett. 2012;10:74-75.
29. Lockhart PB, Loven B, Brennan MT, Fox PC. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J Am Dent Assoc. 2007;138:458-474.
30. Berbari EF, Osmon DR, Carr A, et al. Dental procedures as risk factors for prosthetic hip or knee infection: a hospital-based prospective case-control study. Clin Infect Dis. 2010;50:8-16.
31. American Academy of Orthopaedic Surgeons and American Dental Association. Prevention of orthopaedic implant infection in patients undergoing dental procedures: evidence-based guideline and evidence report. AAOS clinical practice guideline unit. Available at: https://www.aaos.org/Research/guidelines/PUDP/PUDP_guideline.pdf.