You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
With extremely precise cutting ability, minimal collateral damage caused to adjacent healthy tissue, a clear and bloodless operating field, and relatively slight postoperative pain,1,2 the CO2 laser is an optimal surgical solution for the removal of irritation fibromas. These factors, in combination with the laser’s ability to cut in a non-contact mode, help to reduce the risk of complications typically associated with outpatient treatment.2
Etiology of the Irritation Fibroma
The oral irritation fibroma—also known as a traumatic fibroma, focal fibrous hyperplasia, or fibrous nodule—is the most common soft-tissue exophytic lesion occurring in the oral cavity.1 It is a reactive connective-tissue hyperplasia caused most often by persistent irritation or trauma to the oral mucous membranes. Exuberant fibrous connective-tissue repair leads to the formation of a visible submucosal mass.3,4 Major causes for the lesion are trauma and local irritants (eg, calculi, the edges of orthodontic appliances, restorations, foreign bodies, a habit of lip and cheek biting and sucking, and others).5,6
The irritation fibroma tends to occur in frequently traumatized areas in the oral cavity, such as the lower labial mucosa, the lateral border of the tongue, and, occasionally, the gingiva. Most commonly, however, the lesion is found on the buccal mucosa, along the line of occlusion.4,7 The lesion typically manifests itself as a raised, well-delimited, smooth, firm nodule. It is similar in color to the surrounding healthy mouth lining, but may be more translucent.4,8 If it has bled, the lesion may appear darker. In dark-skinned patients, it may have a gray-brown pigmentation.
Occasionally, continued low-grade friction can lead to hyperkeratosis and cause the lesion to appear paler than the rest of the oral tissues, or even whitish in appearance.4 Most irritation fibromas are sessile, but some may also be pedunculated. Additionally, if it has developed under a denture it may be flat with a leaf-like shape.4 Irritation fibromas develop over weeks or months. They have limited growth potential because of its low mitotic index and they tend not to exceed 1 cm to 2 cm in diameter.4,7,9 Because nerve tissue does not proliferate with reactive hyperplastic tissue, the irritation fibroma is painless.9
The irritation fibroma is mostly seen in older adults 40 to 60 years of age, but it can occur at any age and affects 1% to 2% of all adults.4 No gender or racial predilection for the development of this lesion has been reported.9 The recurrence rate is very low, provided that the lesion is completely removed and the source of persistent trauma is eliminated.7,9 Irritation fibromas do not have malignant potential.6,9
Histology
The submucosal fibroma mass consists of dense collagenized tissue with varying amounts of widely scattered mature fibroblasts. Sparse, mild-to-moderate chronic inflammatory infiltrates (eg, lymphocytes and plasma cells) may be seen, usually in a perivascular distribution.4,8,10 The lesion is covered by stratified squamous epithelium, which is often thinned and hyperkeratotic due to chronic trauma.3,9
Diagnosis
A diagnosis of irritation fibroma is done on the basis of both clinical and histopathological examination. The histopathological testing of the excised specimen is necessary to rule out other pathological processes that can mimic an irritation fibroma.4,11 Depending on the location of the lesion in the oral cavity, several other tumors should be included in a clinical differential diagnosis. Thus, for tumors on the tongue, neurofibroma, Schwannoma, and granular cell tumor should be considered; for tumors located on the lower labial and buccal mucosa, such masses as lipoma, mucocele, and salivary gland tumors should be considered.9
The irritation fibroma is typically a solitary lesion. In cases of multiple lesions, associated diagnoses need to be considered, such as tuberous sclerosis, Cowden syndrome, multiple hamartoma syndrome, familial fibromatosis, and fibrotic papillary hyperplasia of the palate.9
Treatment
It has been histologically proven that the age of the irritation fibroma growth correlates with the lesion’s density. Newer lesions are mostly composed of unpacked collagen, while older ones consist of packed, well-organized collagen.3 Thus, with younger, smaller, and less-dense lesions, the clinician may consider preventive, nonsurgical treatment, presuming that with the elimination of the trauma, the lesion will gradually resolve itself. Preventive treatment implies the exclusion of the source of chronic irritation involved, such as management of a trauma-causing biting habit, wearing a lip bumper, and so on.3,9,11 With older, larger lesions composed of denser connective tissue, the conventional treatment includes both simple surgical excision and preventive measures pertaining to the presenting condition.3,6,7,9,11
Soft-Tissue Laser Surgery
The much-praised clinical benefits and ease of use of surgical and dental lasers are enjoyed by tens of thousands of physicians, dentists, and veterinarians worldwide, as well as their collective millions of patients. The key to successful applications of soft-tissue lasers, and their advantages over other surgical tools,12-35 is their ability to accurately cut and efficiently coagulate the soft tissue at the same time.
However, not all lasers are efficient at both cutting and coagulating. Some laser wavelengths (such as those of Erbium lasers) are great at cutting, but are not efficient at coagulating. Other wavelengths (such as those of diode lasers) are efficient coagulators, but are poor scalpels. There are also lasers (such as the CO2 laser) that are efficient at both cutting and coagulating the soft tissue. The key to understanding how the laser light cuts and coagulates is through the wavelength-dependent nature of laser light’s interaction with the soft tissue; namely, light absorption and light scattering by the soft tissue.
Laser Light Absorption and Scattering by the Soft Tissue
Figure 1 presents the modern understanding36 of how various laser wavelengths interact with the main chromophores (absorption centers) in the oral soft tissue for the three wavelength groups of practical dental lasers that are on the market today:
• circa 1,000 nm (diodes and Nd:YAG laser);
• circa 3,000 nm (Erbium lasers); and
• circa 10,000 nm (CO2 lasers).
Light scattering by the soft tissue is insignificant at Erbium and CO2 laser wavelengths.37-39 Soft-tissue light scattering dominates over absorption at near-infrared diode and Nd:YAG laser wavelengths,36-39 which makes these wavelengths poorly suited for precise ablation, incision, and excision.37-40
Soft-Tissue Laser Ablation
Soft-tissue laser ablation (and incision and excision) is a process of vaporization of intra- and extracellular water heated by the laser light within the irradiated soft tissue.37-39 Water vapors, rapidly steaming out of the intensely laser-heated soft tissue, carry with them cellular ashes and other byproducts of this fast boiling and vaporization process.
Because of weak absorption and strong scattering by the soft tissue, the near-infrared diode and Nd:YAG laser wavelengths circa 1,000 nm are highly inefficient and spatially inaccurate laser ablation tools.37-39 Because of very strong absorption by the soft tissue, mid-infrared Erbium (circa 3,000 nm) and infrared CO2 laser (circa 10,000 nm) wavelengths are highly efficient and spatially accurate laser ablation tools.37-39
Soft-Tissue Laser Coagulation and Hemostasis
Coagulation occurs as the denaturation of soft-tissue proteins occurs in the 60°C to 100°C temperature range, leading to a significant reduction in bleeding as well as oozing of lymphatic liquids on the margins of ablated tissue during laser ablation, excision, and incision procedures. Because blood is contained within and transported through the blood vessels, the diameter of blood vessels (estimated to range from 21 µm to 40 µm, with an average value of 31 µm from measurements in human cadaver gingival connective tissue41) is a highly important spatial parameter that influences the efficiency of coagulation process. Collagen shrinks at increased temperatures, which in turn shrinks blood vessel walls and lymphatic vessels, causing hemostasis during laser coagulation.
For Erbium laser wavelengths circa 3,000 nm, optical absorption and coagulation depths are significantly smaller than gingival blood vessel diameters. Coagulation takes place on a relatively small spatial scale and cannot prevent bleeding from the blood vessels severed during tissue ablation. Coagulation depth can be increased by pulse width/rate increase, and by pulse power/fluence decrease.
For diode and Nd:YAG laser wavelengths circa 1,000 nm, optical absorption and coagulation depths are significantly greater than blood vessel diameters. Coagulation takes place over extended volumes—far away from the intended ablation site where no coagulation is required.
For CO2 laser wavelengths circa 10,000 nm, optical absorption and coagulation depths are of the same order as gingival blood vessel diameters. Coagulation extends just deep enough into a severed blood vessel to stop the bleeding. Coagulation depth can be increased by an increase in pulse width/rate, and by decreasing pulse power/fluence.
Optimal Wavelength for Soft-Tissue Laser Surgery
Wavelengths circa 10,000 nm are >1,000 times superior to wavelengths circa 1,000 nm for soft-tissue ablation, and >10 times superior to wavelengths circa 3,000 nm for soft-tissue coagulation and hemostasis. The wavelengths circa 10,000 nm (eg, the CO2 laser) deliver both soft-tissue ablation and simultaneous coagulation, which is unobtainable with either diodes (circa 1,000 nm) or Erbium (circa 3,000 nm) wavelengths.
Laser Pulsing and Thermal Relaxation Time
The application of laser energy over an extended period of time may result in inefficient tissue cutting because of thermal diffusion of laser-generated heat from the irradiated tissue, which may lead to undesirable tissue necrosis and charring on the margins of the laser incision. Proper laser pulsing is of the utmost importance for the appropriate application of laser energy for soft-tissue laser ablation and coagulation.37-39 The most efficient heating of the irradiated tissue takes place when the duration of the laser pulse is much shorter than the thermal relaxation time (which is laser-wavelength and tissue-type specific); the most efficient tissue cooling takes place if the duration between laser pulses is much greater than the thermal relaxation time.
The so-called “SuperPulse” design38 for CO2 laser pulsing parameters is optimized around the thermal relaxation time concept discussed above, and provides for the char-free soft-tissue ablation, incision, and excision with variable depths of coagulation/hemostasis on the margins of the cut.
The SuperPulse mode (Figure 2) is made with bursts of short laser pulses with very high peak power that are spaced far enough apart for efficient tissue cooling between the pulses. The SuperPulse minimizes the amount of heat diffusing from the cutting/ablation zone to the surrounding tissue.
Nd:YAG Soft-Tissue Ablation and Coagulation
The Nd:YAG laser’s 1,064-nm wavelength is an efficient coagulator but a poor scalpel, as it is highly scattered and weakly absorbed by the soft tissue.37-39 To enhance its cutting efficiency, the low absorption of the Nd:YAG wavelength can be attenuated by the use of very high peak power38 typical for free-running pulsed Nd:YAG lasers. When used in contact mode, the Nd:YAG laser may function as a hot-tip cutting tool.37,38
CO2 Laser Soft-Tissue Surgery
The CO2 laser is used directly to photo-thermally cut, ablate, and, at the same time, photo-thermally coagulate the soft tissues. The key to the success of the soft-tissue CO2 laser is its ability to cut and coagulate the soft tissue simultaneously. The literature suggests that the use of the CO2 laser for the treatment of hyperplastic oral lesions, including the irritation fibroma, presents several advantages over conventional scalpel surgery, such as excision without direct tissue contact (hence no mechanical trauma to the tissue) and without bleeding or the need for sutures, precise tissue removal, and minimized postoperative pain and edema. The use of a CO2 laser on the oral soft tissue has no known contraindications or side effects.24 The excellent hemostatic capacity of the CO2 laser is described as a useful instrument for oral surgery in patients with hemorrhagic disorders or undergoing antithrombotic therapy.21-24
Laser Handpieces
Since the early days of surgical CO2 lasers in the 1970s and 1980s, the articulated arm beam delivery system was a barrier to wide adoption of the technology. The paradigm change for CO2 laser surgery was brought about by the invention of the flexible hollow-fiber CO2 laser beam delivery system in the late 1980s. The modern flexible-fiber CO2 laser handpiece is pen-sized, disposable-free, autoclavable, and easily adaptable to switching back and forth between (1) incision with photo-coagulation; (2) superficial ablation with photo-coagulation; and (3) photo-coagulation.
Tip-retainer laser handpieces use disposable hollow focusing tips made of high-temperature resistant aluminum-oxide ceramic (Figure 3) with a 250-µm spot size to allow for intrasulcular periodontal applications.
Disposable-free “tipless” CO2 handpieces are designed to closely simulate the scalpel-like experience without making any contact with the tissue. Maintaining 1 mm to 3 mm of distance between the distal end of the handpiece and the tissue (Figure 3) is required to achieve the designated spot size for cutting.
Laser Power Density
Consider a steel blade: Regardless of how sharp the blade is, there will be no interaction between the blade and the tissue unless mechanical pressure is applied to the blade, forcing it through the tissue’s surface. For a CO2 laser scalpel, the power density of the focused laser beam is equivalent to the mechanical pressure that is applied to a cold steel blade—the greater the laser power density, the greater the rate of soft-tissue removal.
Laser Beam Spot Sizes for Cutting and Coagulation
Just as the sharpness of the steel blade defines the quality and the ease of the cut, the size of the laser beam focal spot defines the quality of the laser cut. Just as a dull blade cannot produce a quality incision, an oversized laser beam spot cannot produce a good quality incision. The smaller (or sharper) the focal spot of the beam, the narrower and deeper the incision. For a rapid switch from cutting to just photo-coagulation, the laser beam can be defocused by simply moving the handpiece away from the tissue by approximately 6 mm to 9 mm, and “painting” the “bleeder” for enhanced hemostasis (Figure 4 and Figure 5).
Pogrel et al17 observed a relatively narrow, <100-µm, zone of thermal necrosis and a variable zone of reversible thermal changes adjacent to the zone of necrosis (100 µm to 500 µm wide). The authors pointed out that the thermal side effects of the CO2 laser could be controlled and reduced by changing the power setting, pulse duration, and pulse repetition rate. A <50-µm thin coagulation zone thickness was reported by Wilder-Smith et al35 for the flexible fiber CO2 laser at 10,600 nm.
Wound Healing
The ability to provide excellent hemostasis is especially valuable as it allows for more precise and accurate tissue removal because the clinician has improved visibility of the surgical field.25 Because of the hemostatic ability of the CO2 laser, intraoral surgical wounds often can be left to heal by secondary intention without placing sutures or dressing.26
Another advantage of the CO2 laser is minimal postoperative swelling and edema because of the intraoperative closure of lymphatic vessels on the margins of the CO2 laser incision. Lymphatic vessels regenerate successively in approximately 8 to 10 days after capillary-vessel proliferation.2
Among the most important advantages of CO2 laser treatment are significantly reduced wound contraction and scarring.26-30 In CO2 laser-irradiated wounds, the healing process is characterized by a more prominent fibroblastic proliferation, with young fibroblasts actively producing collagen; only a small number of myofibroblasts (the cells responsible for wound contraction) is found in the CO2 laser-excised wounds compared with scalpel wounds.30-32 According to Basu et al33 and Tambuwala et al,34 healing of the wounds caused by the CO2 laser involves the appearance of a fibroserous membrane 72 hours postoperatively. This membrane replaces the superficial necrotic layer of the laser-treated area. An epithelial covering of the wound starts to form from the periphery toward the center after 2 weeks, and is thinner and parakeratotic in comparison with the epithelium that forms after scalpel resection. This could account for the excellent esthetic outcome of all CO2 laser treatment, with no fibrosis or scarring and soft pliable residual tissue, while a scalpel can leave some scarring.29
Reduced wound contraction combined with decreased lateral tissue damage, less traumatic surgery, more precise control of the depth of tissue damage, and excellent hemostatic ability make the CO2 laser a safe and efficient alternative to the conventional scalpel. Strauss et al19 and Deppe et al20 report that the healing process with CO2 laser surgery is faster and less painful than after cryosurgery or electrosurgery.
It should be mentioned, however, that the healing process for surgical laser wounds may be prolonged in comparison with surgical scalpel wounds.20,24 At the same time, Lambrecht et al2 reported a slightly shorter delay of just 3 to 10 days. The key to minimizing the healing time is through minimizing the thermal damage on the margins of laser incision/ablation (eg, utilizing the SuperPulse CO2 laser settings).
CO2 Laser Excision Applicability to Fibroma Removal
The removal of an irritation fibroma can be most efficiently performed with the CO2 laser because of its inherent advantages, such as hemostasis, diminished postoperative edema, uneventful healing, and decreased scarring.28 It is crucial for the clinician to rely on the basic principles of laser surgery physics to use the laser efficiently19 with correct laser parameters (SuperPulse), and the sharpest focal laser spot (250-µm diameter) with the ability to rapidly defocus for enhanced coagulation and hemostasis.
Irritation Fibroma CO2 Laser Excision: Case Study
Initial Findings
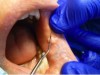
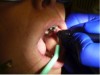
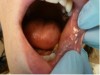
The patient presented with a raised, round, circumscribed lesion on the left buccal mucosa (Figure 6). The lesion was 8 mm in diameter and not ulcerated. The patient stated that the lesion had been present for about 5 years and was slowly increasing in size. The patient requested that it be removed.
Diagnosis and Treatment Plan
The lesion was clinically diagnosed as an irritation fibroma. It was decided to surgically remove the lesion with the CO2 laser. The excised specimen was sent for histopathological analysis.
Surgical Laser Equipment and Settings
A flexible-fiber dental CO2 laser with an autoclavable dental angled tipless handpiece was set to 2 W in the SuperPulse mode with repeat pulsing at a 20-millisecond pulse-width and a 29-Hz repetition rate. The tipless angled handpiece was used at a 1-mm to 2-mm nozzle-to-tissue distance to ensure the 0.25-mm focal-spot size on the target mucosa.
CO2 Laser Surgery
The growth was neatly excised with the laser, providing coagulation of the area as the incision was being made with minimal need for suction. The procedure steps were as follows:
1. Local anesthesia was administered by local infiltration.
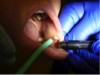
2. A single suture was placed through the lesion to create traction and to ensure complete and uniform removal (Figure 7).
3. The growth was gently pulled distally to provide tension (Figure 7). The initial laser incision was made around the border of the lesion with 1-mm to 2-mm “safety margins” of healthy tissue (Figure 8).
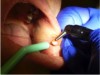
4. Two more laser passes were made to circumscribe the lesion (Figure 9 and Figure 10).
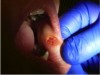
5. With a traction suture pulling the fibroma, the lesion was undermined with the laser beam until excision of the mass was completed (Figure 11). Minor oozing occurred during the procedure. To stop it, pressure was applied for a few seconds using a saline-moistened Q-tip.
6. The laser was used out of focus by moving the handpiece farther away from the surgical area and additional hemostasis was created. No sutures were required and the wound was left to heal by second intention. Note the lack of blood and the cleanly cut area in Figure 8 through Figure 11.
7. The excised specimen, shown in Figure 12, was sent for histopathological examination and the returned report confirmed the initial clinical diagnosis of irritation fibroma.
Postoperative Instructions
Topical antibiotic and vitamin E gel were prescribed to be applied twice daily directly to the area to aid in recovery. The patient was instructed to avoid spicy, acidic, or harsh foods or caustic mouthrinses. The patient reported minimal discomfort during and after the procedure.
Follow-up Examination
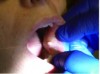
Fourteen days after the CO2 laser surgery, the patient returned for a follow-up visit. Note the clean healing of the buccal mucosa in Figure 13. The recovery was uneventful. The lesion did not recur.
Non-Laser Surgical Treatment Modalities
The existing literature has described several surgical modalities for the treatment of soft-tissue intraoral lesions, including the irritation fibroma, such as scalpel surgery (used most frequently), cryosurgery, electrosurgery, and hot-tip diode excision.
Scalpel Surgery
Surgical enucleation with a scalpel is the most widely used form of treatment, and consists of complete removal of the lesion with safety margins during the surgical procedure, which creates enough material for histopathological testing.7 The obvious drawbacks of conventional scalpel surgery include intraoperative bleeding management, the need for suturing, and the risk of postoperative edema.
Cryosurgery
Cryosurgery uses liquid nitrogen to destruct tissue by rapid freezing. Although it is considered safe for treating oral mucosa lesions, is inexpensive, and has been used successfully for the treatment of the irritation fibroma,14 cryosurgery is time-consuming and has many contraindicated conditions, such as patients with: cold intolerance; cryoglobulinemia; cold urticarial vasculitis; agammaglobulinemia; dysfibrinogenemia; Raynaud’s disease and other collagen diseases; platelet alterations, or are undergoing hemodialysis or immunosuppressive therapy.15 The possible complications include pain during freezing, edema and exudation, vesicles, and bullae. The prolonged complications are hypertrophic scarring and neuropathy; there could be pseudoepitheliomatous hyperplasia, postoperative infection, fever, and pyogenic granuloma.16 In addition, a biopsy specimen for definitive diagnosis has to be obtained prior to cryosurgery and two to three freeze-thaw cycles may be required.15
Electrosurgery
Electrosurgery can be very invasive because of the excessive generation of heat with the potential for scarring. Pogrel et al17 studied the thermal damages in different types of soft tissue. The authors concluded that the relatively narrow zone of thermal necrosis makes the CO2 laser excision superior for histologic examination of excised specimens compared with electrosurgery. Moreover, because of its non-contact cutting ability, CO2 laser surgery is faster than electrosurgery, which requires constant cleaning of the surgical instrument. In addition, the use of electrosurgery may be dangerous or even impossible in patients with metal orthodontic appliances.18
Soft-Tissue Diode/Non-Laser Thermal Ablation and Coagulation
Soft-tissue diode near-infrared laser light circa 1,000 nm is not used to optically ablate the oral soft tissue; instead, the diode laser optical energy is used exclusively to heat up the charred distal end of the fiberglass tip to 500°C to 900°C,42 which then heats up the soft tissue through heat conduction from the hot glass tip. Soft tissue is burned off (ablated) on contact with the hot charred glass tip, while the margins of the burn are coagulated. Unlike non-contact surgical lasers (such as CO2 or Erbium), the soft-tissue ablative diodes are thermal contact, non-laser, wavelength-independent devices.
Conclusion
The 10,600 nm CO2 laser is a highly efficient and spatially accurate photothermal ablation tool with excellent coagulation efficiency because of the close match between coagulation depth and oral soft-tissue blood capillary diameters.
The excision of benign soft-tissue masses, such as the irritation fibroma, with the flexible-fiber waveguide 10,600-nm CO2 laser is a minimally invasive and typically suture-free surgical modality that ensures dependable treatment of fibromas and is, in many respects, superior to most of the alternative treatment options. The CO2 laser is especially well suited for the removal of these lesions because of the excellent hemostasis it provides and the minimal damage it causes to the surrounding tissues.
The extremely precise cutting ability, minimal collateral damage to the adjacent healthy tissue, clear and bloodless operating field and, reportedly, relatively minimal postoperative pain make the CO2 laser an optimal surgical solution to the removal of irritation fibromas. In combination with the laser’s ability to cut in a non-contact mode, the risks of complications typically associated with outpatient treatment are reduced.
Acknowledgments
The authors greatly appreciate the support and contribution from Anna Glazkova, PhD, in preparing this material for publication.
References
1. Haytac MC, Ozcelik O. Evaluation of patient perceptions after frenectomy operations: a comparison of carbon dioxide laser and scalpel techniques. J Periodontol. 2006;77(11):1815-1819.
2. Lambrecht JT, Stübinger S, Hodel Y. Treatment of intraoral hemangiomas with the CO2 laser. J Oral Laser Appl. 2004;4:89-96.
3. Dayan D, Bodner L, Hammel I, Wolman M. Histochemical characterization of collagen fibers in fibrous overgrowth (irritation fibroma) of the oral mucosa: effect of age and duration of lesion. Arch Gerontol Geriatr. 1994;18(1):53-57.
4. Neville BW, Damm DD, Allen CM, Bouquot JE. Soft tissue tumors. In: Neville BW, Damm DD, Allen CM, Bouquot JE, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders; 2009:507-570.
5. Wood NK, Goaz PW. Differential Diagnosis of Oral and Maxillofacial Lesions. 5th ed. St. Louis, MO: Mosby; 2006.
6. Ragneeth BN, Moses J, Reddy VK. A rare presentation of mucocele and irritation fibroma of the lower lip. Contemp Clin Dent. 2010;1:111-114.
7. Valério RA, de Queiroz AM, Romualdo PC, et al. Mucocele and fibroma: treatment and clinical features for differential diagnosis. Braz Dent J. 2013;24(5):537-541.
8. Buchner A, Shneiderman-Shapiro A, Vered M. Relative frequency of localized reactive hyperplastic lesions of the gingiva: a retrospective study of 1675 cases from Israel. J Oral Pathol Med. 2010;39:631-638.
9. Regezi JA, Sciubba JJ, Jordan RCK. Connective tissue lesions. In: Regezi JA, Sciubba JJ, Jordan RCK, eds. Oral Pathology: Clinical Pathologic Correlations. 6th ed. St. Louis, MO: Saunders; 2012:12-85.
10. Kademani D, Bagheri SC. Head and neck pathology. In: Bagheri SC, Jo C, eds. Clinical Review of Oral and Maxillofacial Surgery: A Case-Based Approach. St. Louis, MO: Mosby; 2008:107-141.
11. Sawisch TJ. Oral surgery for the general practitioner: ablation/vaporization techniques and procedures–clinical scenarios. In: Convissar RA. Principles and Practices of Laser Dentistry. St. Louis, MO: Mosby; 2011:93-113.
12. Niccoli-Filho W, Morossoli AR. Surgical treatment of ranula with carbon dioxide laser radiation. Lasers Med Sci. 2004;19:12-14.
13. Zola M, Rosenberg D, Anakwa K. Treatment of a ranula using an Er-Cr:YSGG laser. J Oral Maxillofac Surg. 2006;64(5):823-827.
14. Ishida CE, Ramos-e-Silva M. Cryosurgery in oral lesions. Int J Dermatol. 1998;37(4):283-285.
15. Graham GF. Cryosurgery for benign, premalignant and malignant lesions. In: Wheeland RG, ed. Cutaneous Surgery. Philadelphia, PA: WB Saunders; 1994:835-869.
16. Faber WR. Side effects and complications in cryosurgery. Dermatol Monatsschr. 1993;179:247-251.
17. Pogrel MA, McCracken KJ, Daniels TE. Histologic evaluation of the width of soft tissue necrosis adjacent to carbon dioxide laser incisions. Oral Surg Oral Med Oral Pathol. 1990;70(5):564-568.
18. Convissar RA, Diamond LB, Fazekas CD. Laser treatment of orthodontically induced gingival hyperplasia. Gen Dent. 1996;44(1):47-51.
19. Strauss RA, Fallon SD. Lasers in contemporary oral and maxillofacial surgery. Dent Clin North Am. 2004;48(4):861-888.
20. Deppe H, Horch HH. Current status of laser applications in oral and cranio-maxillofacial surgery. Med Laser Appl. 2007;22(1):39-42.
21. Coleton S. Lasers in surgical periodontics and oral medicine. Dent Clin North Am. 2004;48(4):937-962.
22. Monteiro LS, Mouzinho J, Azevedo A, et al. Treatment of epulis fissuratum with carbon dioxide laser in a patient with antithrombotic medication. Rev Port Estomatol Med Dent Cir Maxilofac. 2011;52:165-169.
23. Tuncer I, Ozçakir-Tomruk C, Sencift K, Cöloğlu S. Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed Laser Surg. 2010;28:75-79.
24. Gama SK, De Araujo TM, Pinheiro AL. Benefits of the use of the CO2 laser in orthodontics. Lasers Med Sci. 2008;23:459-465.
25. Kotlow LA. Lasers in pediatric dentistry. Dent Clin North Am. 2004;48(4):889-922.
26. Zaffe D, Vitale MC, Martignone A, et al. Morphological histochemical and immunocytochemical study of CO2 and Er:YAG laser effect on oral soft tissues. Photomed Laser Surg. 2004;22(3):185-189.
27. Zeinoun T, Nammour S, Dourov N, et al. Myofibroblasts in healing laser excision wounds. Lasers Surg Med. 2001;28:74-79.
28. Mason C, Hopper C. The use of CO2 laser in the treatment of gingival fibromatosis: a case report. Int J Paediatr Dent. 1994;4(2):105-109.
29. Wang X, Ishizaki NT, Matsumoto K. Healing process of skin after CO2 laser ablation at lo radiance: a comparison of continuous-wave and pulsed mode. Photomed Laser Surg. 2005;23:20-26.
30. Grbavac RA, Veeck EB, Bernard JP, et al. Effects of laser therapy in CO2 laser wounds in rats. Photomed Laser Surg. 2006;24(3):389-396.
31. de Freitas AC, Pinheiro AL, de Oliveira MG, Ramalho LM. Assessment of the behavior of myofibroblasts on scalpel and CO2 laser wounds: an immunohistochemical study in rats. J Clin Laser Med Surg. 2002;20(4):221-225.
32. Fisher SE, Frame JW, Browne RM, Tranter RMD. A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch. Oral Biol. 1983;28(4):287-291.
33. Basu MK, Frame JW, Rhys Evans PH. Wound healing following partial glossectomy using the CO2 laser, diathermy and scalpel: a histological study in rats. J Laryngol Otol. 1988;102(4):322-327.
34. Tambuwala A, Sangle A, Khan A, Sayed A. Excision of oral leukoplakia by CO2 lasers versus traditional scalpel: A comparative study. J Maxillofac Oral Surg. 2014;13(3):320-327.
35. Wilder-Smith P, Arrastia AM, Liaw LH, Berns M. Incision properties and thermal effects of three CO2 lasers in soft tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):685-691.
36. Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58(11):R37-R61.
37. Fisher JC. Basic laser physics and interaction of laser light with soft tissue. In: Shapshay SM. ed. Endoscopic Laser Surgery Handbook. New York, NY: Marcel Dekker; 1987:96-125.
38. Fisher JC. Qualitative and quantitative tissue effects of light from important surgical lasers. In: Wright CV, Fisher JC, eds. Laser Surgery in Gynecology: A Clinical Guide. Philadelphia, PA: Saunders; 1993:58-81.
39. Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103(2):577-644.
40. Willems PWA, Vandertop WP, Verdaasdonk RM, et al. Contact laser-assisted neuroendoscopy can be performed safely by using pretreated ‘black’ fibre tips: Experimental data. Lasers in Surgery and Medicine. 2001;28(4):324-329.
41. Yoshida S, Noguchi K, Imura K, et al. A morphological study of the blood vessels associated with periodontal probing depth in human gingival tissue. Okajimas Folia Anat Jpn. 2011;88(3):103-109.
42. Romanos G. Diode laser soft-tissue surgery: Advancements aimed at consistent cutting, improved clinical outcomes. Compend Cont Educ Dent. 2013;34(10):752-758.
About the Authors
Robert Levine, DDS
Director of Laser Dentistry
Arizona School of Dentistry & Oral Health
Mesa, Arizona
Founder
Global Laser Oral Health LLC
Scottsdale, Arizona
Peter Vitruk, PhD, MInstP, CPhys
Founder
LightScalpel LLC
Woodinville, Washington
Faculty
California Implant Institute
San Diego, California
Faculty
Global Laser Oral Health LLC
Scottsdale, Arizona