You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Complications attributed to moderate and deep levels of sedation are more often associated with respiratory compromise and have been reviewed in a previous article.1 Sedation, and general anesthesia for that matter, have minimal influence on overall cardiovascular function in reasonably healthy patients. In fact, cardiovascular complications may be even more likely during dental procedures using local anesthesia alone than when sedation is provided. However, respiratory compromise, excessive drug dosages, or inadequate anesthesia may trigger cardiovascular events, and the well-informed provider should be familiar with their recognition and management.
ABNORMALITIES IN ARTERIAL BLOOD PRESSURE
Neurocardiogenic (Vasovagal) Syncope
Syncope is a transient loss of consciousness due to reduced cerebral blood flow. It may occur unexpectedly but is often preceded by such signs and symptoms as pallor, lightheadedness, diaphoresis, and nausea. As brain tissues are deprived of oxygen, a brief period (ie, a few seconds) of convulsive motor activity is not unusual during a syncopal episode, especially if the patient remains in an upright posture. This event is frequently confused as a primary seizure event.
Although syncope may be the result of a cardiac arrhythmia, in dental practice it is most often the result of what is commonly referred to as a vasovagal event. Vasovagal syncope is associated with an eventual loss of sympathetic tone (vasodilatation) and increased parasympathetic activity (bradycardia and gastrointestinal stimulation). The term neurocardiogenic syncope is used to encompass both vasovagal syncope and vasodepressor syncope in which only loss of sympathetic tone occurs.2 In either event, the syncopal episode is triggered by painful or stressful events not uncommon during dental procedures.
The pathogenesis of vasovagal syncope commences with increased peripheral sympathetic activity and venous pooling. A decline in venous return leads to forceful myocardial contractions of the left ventricle. This in turn activates myocardial mechanoreceptors and vagal afferent nerve fibers that inhibit sympathetic activity and increase parasympathetic activity. These events culminate in bradycardia, vasodilation, and the decline in blood pressure responsible for loss of consciousness.2 Indeed, it is not unusual for a syncopal episode to be preceded by a brief period of forceful pounding of the heart and tachycardia, which contribute to the subsequent neural reflexes leading to the vasovagal event.
The depth and duration of unconsciousness during vasovagal syncope are highly variable. In some cases, vagal influences are severe enough to induce transient periods of asystole that persist for 30 to 40 seconds.3,4 Its management should be well understood by all dentists, whether or not sedation is being administered. This consists of carrying out the primary assessment and management of airway, breathing, and circulation while positioning the patient supine with legs elevated. Regardless of the cause or severity, vasovagal events will generally subside during the time primary measures for assessment and airway support are instituted. Subsequently, attention must be directed toward abnormalities in blood pressure and heart rate that may or may not require pharmacologic intervention. Syncope that does not resolve spontaneously or with minor intervention is unlikely vasovagal in mechanism, and other causes, such as cardiac arrhythmia, stroke, and drug overdose, should be explored.
Hypotension
Episodes of hypotension in clinical practice are most commonly associated with vasovagal events and are generally transient, but they may become prolonged in the presence of central nervous system depressants. The same can be said for postural (orthostatic) hypotension, which normally subsides with proper repositioning of the patient. The blood pressure required to perfuse tissues adequately varies from patient to patient and is influenced by their medical status and posture at the time of assessment.
A significant decline in blood pressure from baseline should alert the clinician, but hypotension cannot be established on precise numerical values alone. Evaluation of tissue perfusion is the more significant component of cardiovascular assessment. Color changes in the skin and mucosa and the rate of capillary refill subsequent to squeezing of the nail beds can be used as a guide for assessing perfusion of peripheral tissues. The adequacy of perfusion within the central nervous system can be estimated by the patient’s response to verbal and painful stimuli in the conscious patient or by pupillary reflex when they are unconscious or heavily sedated. If blood pressure has declined and perfusion is considered inadequate, the clinician may elect to increase blood pressure. To do this appropriately, several physiologic principles must be considered.
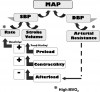
Blood pressure fluctuates continuously due to the cyclic nature of the pumping action of the heart. The highest pressure is produced by ventricular contraction (systole) and is designated systolic blood pressure. The lowest pressure occurs during ventricular relaxation (diastole) and is therefore designated as diastolic blood pressure. This is the result of arterial resistance. Mean arterial pressure is the time-weighted average of the blood pressure throughout the cardiac cycle and is an indication of adequacy or inadequacy of perfusion.
One must avoid excessive elevation of arterial resistance and diastolic pressure, because it can produce undo strain on the heart. For the heart to eject a stroke volume, the left ventricle must generate a pressure that exceeds peripheral resistance. In other words, ventricular pressure must exceed diastolic pressure. This resistance to ventricular ejection is called afterload, and for a patient with heart disease, elevated diastolic pressure not only stresses the heart but may also hinder ejection of an adequate stroke volume. On the other hand, coronary artery blood flow occurs during diastole when the heart muscle relaxes, so a reasonable diastolic blood pressure must be present for the heart to be nourished before the next systolic contraction occurs. However, diastolic blood pressures of 30 mm Hg are generally adequate for this purpose for most adults. In the primary care setting, systolic blood pressure is the target of monitoring and interventions.
Systolic blood pressure is primarily a function of cardiac output, which is calculated as ventricular rate multiplied by the stroke volume. Of these two factors, stroke volume is most significant in adults because it provides the ‘‘surge’’ that creates the systolic pressure. Except for small children and infants, the heart rate acts merely as a ‘‘compensator’’ for changes in stroke volume. For example, slow rates are common in well-trained athletes, but rapid rates are required to sustain adequate cardiac output for patients having low stroke volumes due to heart failure. Therefore, when managing a hypotensive patient, our primary goal is to improve stroke volume.
Stroke volume can be increased in two manners: (a) by improving myocardial contractility, which is augmented by sympathetic stimulation of beta-1 receptors, and (b) by increasing venous return to the heart (preload). According to the Frank-Starling law, preload is directly related to stroke volume, but there is a limit to this relationship. If a critical preload volume is exceeded, congestion occurs. This volume is lower for patients having compromised cardiac function and should be considered when positioning a patient. Although in the past the Trendelenburg position had been cited as the preferred position for patients experiencing medical emergencies, it may allow excessive venous return (increase preload) and compromise patients with cardiac or respiratory disease. In fact, it appears this position offers few advantages.5,6 Thus, the more appropriate position when managing most medical complications in sedated patients or those who are unresponsive is to have the patient supine with the legs elevated slightly. Improving venous return will also increase heart rate according to the Bainbridge reflex. When the right atrium is stretched by venous return, it is sensed by receptors in the area of the sinoatrial node, leading to an increase in heart rate to better accommodate the returning volume. Figure 1 summarizes and illustrates the influences of various parameters on arterial blood pressure.
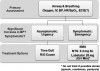
In general, a systolic blood pressure of 90 mm Hg should sustain mean arterial pressure sufficiently to perfuse tissues in the recumbent patient. A pressure lower than this combined with evidence of inadequate perfusion requires intervention. This can be accomplished in the following manners: (a) improve venous return by positioning the patient, administering intravenous fluid, or administering drugs that provide venoconstriction to increase venous pressure and preload; (b) increase myocardial contractility (inotropy) using drugs that activate beta-1 receptors on myocardial cells, providing a positive inotropic influence.
If repositioning of the patient to improve venous return, such as described above, fails to improve the situation, subsequent intervention should proceed in the following manner. If an intravenous line is in place or can be established readily, 250 mL to 500 mL of physiologic solution, such as normal saline, should be infused rapidly unless congestive heart failure is suspected. Generally, this will increase preload sufficiently to improve stroke volume and raise systolic pressure. When this maneuver cannot be accomplished or proves unsuccessful, the patient’s heart rate should guide further treatment. If bradycardia is present, that is, <60 beats/min, administer intravenous atropine until the rate is within normal limits. This is accomplished in 0.5-mg increments. Suggested dosages for medications addressed throughout this article are summarized in Table 2. If the rate is >60 beats/min and pressure remains low, increasing the rate further may do little to improve systolic pressure. As heart rate increases, the time allocated for diastolic filling and each subsequent stroke volume will decline.
Although several adrenergic drugs may be acceptable to manage hypotension, ephedrine is often an ideal choice for several reasons. Hypotension encountered during dental practice is usually attributed to either vasovagal episodes or the use of sedatives and anesthetics that depress sympathetic outflow to the cardiovascular system. (In Advanced Cardiac Life Support courses, however, hypotension is generally cardiogenic and requires more powerful inotropics, such as dopamine and epinephrine.) In either case, ephedrine specifically counters these influences indirectly by stimulating norepinephrine release from sympathetic nerve endings. Also, ephedrine acts directly on alpha- and beta-adrenergic receptors, leading to vasoconstriction and increased rate and contractility of the myocardium. Ephedrine constricts veins to a greater extent than arteries, which enables it to increase preload more than afterload.7 This results in less of an increase in myocardial oxygen demand compared with other vasopressors. Finally, unlike epinephrine and other catecholamines having brief durations of action, that is, 5 to 10 minutes, the cardiovascular effects of ephedrine continue for 60 to 90 minutes. Ephedrine can be administered intravenously in 5-mg to 10-mg increments or 25 mg by sublingual or intramuscular injection. Exceeding a total dose of 50 mg is not recommended.
When it is secondary to hypovolemia, hypotension can be accompanied by tachycardia, so the cardiotonic effects of ephedrine may be undesirable. This situation occurs most often when hypotension is the result of spinal anesthesia, hypovolemia, or dehydration and is unlikely in the dental setting, where hypotension is generally either vagal induced or attributed to drugs that depress the central nervous system. However, patients who have been without oral food and fluids for extended periods may present with a relative hypovolemia secondary to dehydration.
Phenylephrine is an alpha-adrenergic agonist that is useful for treating hypotension when tachycardia is present or when any increase in heart rate should be avoided, such as a patient with significant coronary artery disease. Phenylephrine produces venoconstriction, improving preload and systolic pressure, and produces arterial constriction, which increases diastolic pressure. The elevation in mean arterial pressure may trigger a baroreceptor-mediated reduction in heart rate.7 Phenylephrine is typically administered by continuous intravenous infusion or in 0.1-mg intravenous increments. Its use should be accompanied by adequate fluid administration to ensure that hypovolemia is not present. The use of phenylephrine is best reserved for those with training in deep sedation and general anesthesia. An algorithm approach for managing hypotension is presented in Figure 2.
Hypertension
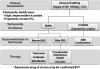
Sudden elevations in blood pressure are not that uncommon in dental practice, regardless of whether sedation is being provided. Precise blood pressures of significance are not defined, but sudden elevations to systolic blood pressure >180 mm Hg or diastolic blood pressure >110 mm Hg are generally regarded as an acute hypertensive episode, keeping in mind that this suggestion fails to consider the patient’s baseline readings.8 In patients with chronic hypertension, autoregulation of cerebral blood flow is reset to a higher level, and abruptly lowering pressure can lead to cerebral ischemia. This is particularly true for geriatric patients.9
A hypertensive episode is generally regarded as urgent when the patient remains asymptomatic and rarely requires treatment other than a ‘‘time out’’ to calm down. These episodes are most likely attributed to waning local anesthesia, a need to use the restroom, or restlessness during lengthy procedures. Gallagher summarized this issue vividly in an article for physicians in the emergency department. ‘‘The most sensible approach to the patient in the [emergency department] found to have very high blood pressure, without evidence of acute end organ damage, is referral for outpatient management of serious disease that needs to be treated, not urgently, but for life. Focusing on the height of the column of mercury in the sphygmomanometer confers no demonstrable benefit on the patient and risks doing harm.’’10
Simply stated, patients without acute end-organ symptoms should not receive antihypertensive agents in the office, and they may be safely referred to their primary physician for follow-up within several days.11-13
Hypertensive emergency and crisis are terms used to describe an acute hypertensive episode accompanied by symptoms of end-organ damage. The event includes chest pain, headache, or visual disturbances. In this case, emergency medical service transport should be arranged immediately. While awaiting emergency medical service transport arrival, any decision to lower the blood pressure is based on the judgment and experience of the provider. If treatment is elected, the goal is to lower the blood pressure by ~20% within 30 to 60 minutes.12-14 However, if signs or symptoms are consistent with stroke, it is wise to avoid any intervention and continue to support the patient while awaiting emergency medical service transport arrival. Clinically, one cannot ascertain if injury is hemorrhagic or infarction, and ischemic brain may be critically dependent on collateral perfusion pressure. Any reduction in blood pressure could prove catastrophic. In this scenario, only extremely high systolic blood pressure (>220 mm Hg) or diastolic blood pressure (>120 mm Hg) should even be considered for treatment.15
A variety of drugs and drug classes are suggested for management of hypertensive emergencies. However, nitroglycerin and labetalol are consistently mentioned and are adequate for office preparedness. Nitroglycerin is a vasodilator that acts predominantly on the venous system, decreasing venous return to the atria and ventricles. At conventional doses, nitroglycerin has little effect on arterial resistance and lowers blood pressure by reducing preload and subsequent cardiac output. This action may be undesirable in patients with impaired cerebral perfusion and should not be used if stroke is suspected. However, it is ideal for those with cardiac symptoms. Nitroglycerin may be administered either sublingually or intravenously, but the former route is most appropriate in the office setting. A single 0.4-mg tablet can be placed sublingually and repeated every 5 minutes while checking blood pressure response before each subsequent dose. Caution is advised when administering more than 1 dose because the incidence of headache may become problematic. In any case, no more than 3 doses should be considered.
Labetalol (Trandate and Normodyne) is a selective alpha-1 blocker and nonselective beta-blocker with a ratio of alpha/beta blockade of ~1:5. Labetalol lowers blood pressure by blockade of the alpha-1 receptors in vascular smooth muscle and the beta-1 receptors in the heart. Because of the simultaneous beta-receptor blockade, the usual reflex tachycardia associated with other vasodilators does not occur. It can be administered in 5-mg to 20-mg increments, depending on the response to the initial dose, while checking blood pressure before each subsequent dose. Patients under sedation or general anesthesia may be more susceptible to the lower dose range. The expected onset of action of intravenous labetalol is 5 minutes. The maximum dose is 0.5 mg/kg or 300 mg, but this amount will rarely be required. When administered in this manner, labetalol is safe with minimal adverse reactions.11 Like all nonselective beta-blockers, labetalol is contraindicated in patients with chronic obstructive pulmonary disease or asthma. Blockade of beta-2 receptors on bronchial smooth muscle may result in bronchoconstriction. Labetalol should be used only by those with advanced training and only when continuous electrocardiographic (ECG) monitoring and minute-by-minute blood pressure recording are in place.
Any drug that lowers blood pressure increases a patient’s risk for orthostatic (postural) hypotension. Due to its longer duration of action, labetalol carries a greater risk than nitroglycerin and patients should be ambulated with great caution. This concern is unlikely in the office setting because any indication for using labetalol is likely accompanied by the need for emergency medical service transport. An algorithm approach for managing acute hypertensive events is presented in Figure 3.
ABNORMALITIES IN THE HEART
Cardiac Dysrhythmias
Most dentists have witnessed a patient complaining of cardiac palpitations after administration of a local anesthetic with epinephrine or during forceful procedures. Frequently this is the result of benign dysrhythmias, such as extrasystoles (premature contractions), but they pass unnoticed because continuous ECG monitoring is usually not in place. Published guidelines for patient monitoring during sedation are consistent in requiring continuous assessment of oxygenation by pulse oximetry. This also provides continuous monitoring of pulse rate but not the specific rhythm, and guidelines for the use of ECG monitoring are less consistent. While stating that moderate and even deep sedation have minimal impact on cardiovascular function, the American Society of Anesthesiologists guidelines nevertheless require ECG monitoring for even moderate levels of sedation.16 This has no evidence-based scientific basis, but from the society’s perspective, it is understandable. All monitoring systems used by anesthesiologists include electrocardiography, and any case scheduled initially for sedation may require instant conversion to a full general anesthetic. However, this requirement may be excessive for the dentist providing only moderate sedation, and guidelines published by the American Dental Association are more practical for the average moderate sedation-trained dentist.17 These guidelines do, however, require continuous ECG monitoring for deep sedation and general anesthesia, but its use for moderate sedation is suggested only for patients having significant cardiovascular disease. This would include patients who have known rhythm disturbances, including those managed with implanted pacemakers.
Legal controversies aside, there is an intangible reassurance provided by an ECG monitor that adds to that provided by periodic measurement of blood pressure and continuous pulse oximetry. This of course presumes the operator understands and can interpret electrocardiograms and is comfortable witnessing occasional benign dysrhythmias and the subtle mechanical nuances all monitors present during routine use. The precise interpretation of a particular dysrhythmia is not as important as recognizing that a disturbance is occurring and deciding whether it is compromising the patient’s hemodynamic status. This will be our approach when addressing selected dysrhythmias and their management. A review of basic electrocardiogram interpretation has been published in a previous article.18
Supraventricular Bradydysrhythmias
Bradycardia is defined as a heart rate of <60 beats/min, but symptoms normally do not arise unless the rate falls to <50 beats/min. Supraventricular bradydysrhythmias may be sinus or junctional in origin, or they may be caused by various degrees of atrioventricular block. In most cases, these events are vagally induced, but central nervous system depressants are potential culprits and may potentiate vagal activity when present. Hypoxemia can also be a common cause, and efforts should be made to investigate and correct this possibility.19 The precise diagnosis of the arrhythmia is not so important as evidence of hemodynamic compromise (hypotension) or ventricular ectopy (premature ventricular contraction [PVC]) due to ventricular escape. When either of these events is present, the bradycardia should be treated with atropine, as previously explained in the discussion of hypotension in this article.
If a bradyarrhythmia does not respond after two doses of atropine, a second or third degree atrioventricular block should be investigated. Second-degree type II and third-degree blocks are located below the atrioventricular node, where there is no parasympathetic innervation. Atropine is seldom effective for infranodal block, and these cases require EMS transport for eventual pacemaker insertion. If hypotension remains significant while awaiting EMS transport, the recommended treatment is epinephrine by continuous infusion titrated from 2 µg/min to 10 µg/min.19 This can be accomplished by adding 1 mL of 1:1,000 epinephrine to a 500-mL bag of normal saline or 5% dextrose, which provides a concentration of 2 µg/mL. Titration with an infusion pump should commence at 1 mL/min with incremental increases guided by blood pressure and heart rate. An epinephrine infusion should be used only by those with advanced training and when continuous ECG monitoring and minute-by-minute blood pressure recording are in place.
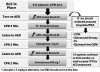
Sinus and Supraventricular Tachydysrhythmias
Tachycardia is defined as a heart rate >100 beats/min, but usually it is not until rates exceed 150 that patients become symptomatic. Transient episodes of tachycardia are triggered most often by pain, stress, and vasopressors included in local anesthetic solutions. It may be an indication that the sedation being administered is not sufficient to manage the patient’s fear. It is important to establish whether the tachycardia is secondary to pain, stress, or vasopressors or whether the tachycardia is truly cardiogenic, which may lead to hypotension or myocardial ischemia. This tachycardia is almost always controlled by the sinoatrial node and is called sinus tachycardia; a P wave precedes each QRS complex. Sinus tachycardia can also be an initial reflex response to hypoxia or hypotension, and these should be considered before further treatment. Once these possibilities have been attended, persistent tachycardia may cause the patient to complain of palpitations. In this case, intravenous fluids should be administered to support blood pressure in the event the rapid heart rate is attempting to sustain the blood pressure in a hypovolemic patient. If the episode continues, a selective beta-1 receptor antagonist, such as esmolol (Brevibloc), can be administered. Due to its relatively brief duration of action (T½ ~9 minutes), esmolol is generally administered as a bolus of 0.5 mg/kg over 2 minutes followed by a continuous intravenous infusion. For office use, we suggest it be titrated intravenously in 20-mg increments every 2 to 3 minutes until the heart rate declines to an appropriate level. There is no maximum dose published for esmolol, but 80 mg to 100 mg is a reasonable limit before determining it is ineffective. If the tachycardia does not respond to this dosage or recurs after the effects of esmolol have waned, EMS transport should be considered. Compared with nonselective beta-blockers, such as labetalol, esmolol is less likely to produce bronchospasm, but it must nevertheless be used with caution in patients with chronic obstructive pulmonary disease or asthma. Esmolol should be used only by those with advanced training and only when continuous ECG monitoring and minute-by-minute blood pressure recording are in place.
Sinus and supraventricular tachydysrhythmias are distinguished from ventricular dysrhythmias by narrow QRS complexes. If P waves are not evident preceding the QRS, the rhythm is not sinus in origin and is described as supraventricular. These dysrhythmias include atrial flutter, atrial fibrillation, and supraventricular tachycardia. It must be emphasized that atrial flutter and fibrillation are fairly common chronic conditions that are usually well tolerated due to medications that control ventricular rate. They introduce concern only when ventricular rate accelerates or they present as a new onset.
In contrast to atrial flutter and fibrillation, supraventricular tachycardia is not a chronic condition, although a patient may have a history of paroxysmal episodes. An onset of supraventricular tachycardia is always a concern. It is generally more rapid, producing heart rates >150 beats/min, and can be distinguished from the others by its regular ventricular rhythm (ie, regular R to R intervals). A precise diagnosis can be challenging, but the important feature determining a need for treatment is hemodynamic instability, that is, hypotension. Although adenosine is regarded as the drug of choice for confirmed supraventricular tachycardia, a beta-blocker, such as esmolol, is also an option.19 Furthermore, esmolol is also an option in treating symptomatic atrial flutter and fibrillation. For this reason, esmolol is an acceptable choice for managing any of the atrial tachyarrhythmias. Compared with sinus tachycardias, any of the supraventricular dysrhythmias that become symptomatic are a far greater concern, and EMS transport should be arranged as treatment is rendered.
Extrasystoles (Premature Contractions)
Extrasystoles are ectopic impulses that occur in addition to the underlying rhythm and occur in most individuals. By convention, the term contraction is applied to these extra impulses, although a true mechanical contraction may not always occur. The source of these ectopic impulses can be anywhere throughout the atria and ventricles: premature atrial contractions, premature junctional contractions, or PVCs. In general, they are all relatively benign rhythms, and any new onset may reflect some sort of stress response that should be addressed.
PVCs are distinguished from other extrasystoles by their bizarre and widened QRS complex. They are generally benign, but when they occur with considerable frequency, they do have a greater potential for complications than other extrasystoles. They may produce hemodynamic instability or lead to lethal dysrhythmias, such as ventricular tachycardia and fibrillation. The Lown criteria are used to classify PVCs according to their frequencies and patterns (Table 1).20 After myocardial infarction, Lown classes 3 to 5 have been found to be associated with a greater risk for conversion to lethal dysrhythmias, but this correlation has not been established for other patients.20 Nevertheless, a reasonable caveat is that treatment is unlikely necessary for PVCs having a uniform appearance, regardless of frequency, unless the patient becomes hypotensive. For classes 3 to 5, treatment and EMS transport are reasonable decisions.
There are many antidysrhythmic drugs advocated for treating patients with PVCs, but lidocaine is the safest and least sophisticated to administer. It can be administered as a single 0.5-mg/kg to 1-mg/kg dose and repeated every 5 minutes up to 3 mg/kg. Its influence will generally last 15 to 20 minutes, after which a continuous infusion of 1 mg/kg to 2 mg/min is required if the condition persists. However, when a patient remains symptomatic after 1 or 2 incremental doses or the condition recurs after its effects have waned, EMS transport should be arranged.
Before electing to use lidocaine for PVCs, it is important to confirm that the underlying rhythm is not a bradycardia, in which the PVCs represent efforts at ventricular escape. In this case, atropine should be administered to increase the underlying heart rate. The ventricular escape beats will then hopefully subside.
Ventricular Tachycardia
Unlike ventricular fibrillation, ventricular tachycardia may not be accompanied by cardiac arrest. It can be distinguished from atrial tachydysrhythmias by wide QRS complexes and the absence of atrial waveforms. It is not uncommon for patients to remain relatively stable while experiencing this dysrhythmia, but it can deteriorate rapidly to cardiac arrest. EMS transport should be summoned immediately. Current advanced cardiovascular life support guidelines suggest procainamide, amiodarone, and sotalol as preferred agents, but lidocaine is still regarded as an acceptable alternative and can be administered in the identical regimen addressed for management of PVCs. If the patient develops chest pain or becomes hypotensive, synchronized cardioversion is recommended if available. The office team should prepare for cardiac arrest, should it occur. Figure 4 provides an algorithm approach for managing atrial and ventricular tachydysrhythmias.
Chest Pain: Angina/Myocardial Infarction
When any of the previously discussed complications become extremely severe, they can either strain the heart or compromise coronary perfusion to the point that the patient may experience an episode of angina pectoris. However, this form of chest pain is most likely to occur if the patient has preexisting coronary artery disease. The event may represent an episode of stable angina or a more serious event labeled acute coronary syndrome. To understand this difference, a basic understanding of the pathogenesis of coronary artery disease must be appreciated.
The fundamental defect in coronary artery disease is stenosis, or narrowing of the lumen of coronary arteries due to atherosclerosis. The condition is not acutely life threatening so long as the lesion remains stable and does not rupture. The patient may experience chest pain if cardiac stress suddenly increases because coronary ‘‘supply’’ is outweighed by myocardial oxygen demand. These episodes of chest pain are regarded as ‘‘stable angina’’ and can be precipitated by the stress of dental treatment. The angina will dissipate when cardiac stress is reduced by calming the patient and perhaps administering a dose of nitroglycerin.
A more serious consequence occurs when atherosclerotic lesions become unstable and rupture, producing the so-called ‘‘acute coronary syndrome.’’ In this case, coronary perfusion becomes even further compromised. Added to the preexisting stenosis, debris from fractured atherosclerotic plaques obstructs coronary flow more severely and subsequent chest pain is described as unstable angina. This form of angina can occur at rest or with stress and may evolve to thrombosis with total occlusion of a coronary vessel. If this occurs, myocardial cells will undergo necrosis, defined as myocardial infarction. Therefore, the acute coronary syndrome is produced by unstable atherosclerotic lesions and manifests as either unstable angina or myocardial infarction, described collectively as acute coronary syndrome.
The dentist can do little to improve coronary blood flow. Patient management must be devoted to reducing cardiac stress and subsequent myocardial oxygen requirement, which hopefully will render the compromised coronary perfusion adequate. When a patient experiences chest pain, a complete primary assessment that includes not only blood pressure and heart rate but also hemoglobin saturation via pulse oximetry should be performed. This will assure that adequate oxygenation is present. Regardless of these results, supplemental oxygen should be provided via nasal cannula (4 L/min) or nasal hood (6 L/min). Any benefit of supplemental oxygenation has not been established for patients who sustain normal hemoglobin saturation on room air, but short-term administration has no adverse effects. Comforting the patient may reduce stress-induced increases in heart rate and blood pressure. If pain persists, nitroglycerin (either a 0.3-mg or 0.4-mg tablet or a 0.4-mg spray) should be administered sublingually, provided that the systolic blood pressure is >90 mm Hg. Nitroglycerin dilates systemic veins and reduces venous return, that is, preload. This reduction in diastolic wall tension or stress may also allow improved coronary perfusion, especially in the subendocardial regions. Nitroglycerin can be repeated every 5 minutes until symptoms improve or side effects (eg, hypotension, reflex tachycardia) occur. Hypotension is particularly troublesome because it could compromise coronary perfusion further and reflex tachycardia increases myocardial oxygen demand. Although reclined patients are not likely to experience these problems, blood pressure and pulse should be assessed before administering each subsequent dose of nitroglycerin.
Nitroglycerin is contraindicated if the patient has taken an erectile dysfunction agent, such as sildenafil (Viagra) and vardenafil (Levitra), within 24 hours or within 48 hours if tadalafil (Cialis) is used.21 Furthermore, we must reemphasize the importance of assessing blood pressure before administering nitroglycerin to any patient. In some cases of myocardial infarction, nitroglycerin can produce a more significant drop in blood pressure. This is particularly true if the infarction involves the inferior wall and right ventricle.22,23 Although this diagnosis requires 12-lead ECG analysis, successful management of these particular infarctions is dependent on improving preload, which is reduced by nitroglycerin. Systolic blood pressure >90 mm Hg should be confirmed before administering each dose.
The actual criteria for need and timing for activation of EMS transport are not well established. The package inserts for nitroglycerin formulations instruct patients with angina pectoris to access EMS transport when three doses of nitroglycerin over 15 to 20 minutes fail to relieve symptoms. Pollack and Braunwald24 have suggested that EMS transport is indicated after administration of three doses of nitroglycerin over a 15- to 20-minute period for stable angina but only one dose if angina is deemed unstable. Current American Heart Association guidelines25 address only suspected acute coronary syndrome (unstable angina or myocardial infarction) and encourage immediate EMS transport. They do not address stable angina. However, it may be impossible for the dentist to ascertain if the condition represents a stable or unstable event, and personal judgment must be used regarding subsequent action. For a patient with preexisting coronary disease, chest pain provoked by a particularly stressful intervention may well represent a typical episode of stable angina. In this case, the patient will respond nicely after a primary assessment or a single dose of nitroglycerin and could very well be sent home after the dental treatment is completed. In contrast, patients having no prior history of angina or who require more than their usual dose to relieve symptoms should be transported to an emergency department for further evaluation. In all cases, it is professionally courteous to inform the patient’s primary physician when possible.
With activation of EMS transport, the decision has been made that the condition is possibly an acute coronary syndrome, and aspirin (300 mg) should be administered. This is accomplished ideally by chewing and swallowing either three or four chewable, flavored baby aspirins (81 mg each) or a regular 325-mg tablet. Platelet aggregation is a key factor during coronary thrombosis, and the maximum antiplatelet influence of aspirin is achieved within 1 hour of administration. Nitroglycerin can be continued every 5 minutes, provided systolic pressure is at least 90 mm Hg and the heart rate is within normal limits.
If pain is severe and persistent, an opioid (narcotic) can be administered. Opioids not only relieve pain and anxiety but also reduce peripheral resistance (afterload) and venous capacitance (preload). This reduces myocardial oxygen demand, that is, a nitroglycerin-like effect. Although morphine is ideal and thus the conventional agent recommended, fentanyl and nalbuphine are acceptable alternatives. Opioids are more likely to produce hypotension if nitroglycerin has been administered, and the clinician should monitor blood pressure carefully and often. An opioid should be considered only if an intravenous infusion is in place and the clinician is familiar with its use. A suggested algorithm for management of chest pain is provided in Figure 5.
Cardiac Arrest
Cardiac arrest is the absence of a pulse. In the office setting, the ECG status will most likely commence with ventricular tachycardia, which deteriorates to ventricular fibrillation. This can subsequently deteriorate further to asystole or pulseless electrical activity. Cardiac arrest is most often attributed to myocardial infarction but may also be triggered by other factors, such as sustained hypoxemia due to severe respiratory depression or airway obstruction.
Once primary assessment confirms cardiac arrest, EMS transport must be obtained immediately and the office team should commence cardiopulmonary resuscitation following the 2010 American Heart Association guidelines as instructed in all healthcare provider courses in basic life support.26 An exemplary office protocol is presented in Figure 6. Ventilations should be performed using a bag-valve-mask device (eg, Ambu-Bag) attached to a 100% oxygen source. Chest compressions must be rapid (100/min) with pauses after 30 compressions to allow for two adequate ventilations. There is little excuse for the entire office staff not being certified in basic life support at the healthcare provider level on a regular basis (ideally on an annual basis). Definitive treatment requires electrical defibrillation as soon as it is available. The beneficial role of cardiopulmonary resuscitation likely rests in its modest influence on coronary perfusion and minimizing hypoxemia, which may sustain electrical activity until defibrillation is available. Supporting this concept are data illustrating greatest success when cardiopulmonary resuscitation is initiated immediately and is followed by defibrillation within 5 to 8 minutes of cardiac arrest. For offices equipped with automated external defibrillators, the device should be turned on and its instructions followed. If an automated external defibrillator is not available, the office team should concentrate on proper delivery of cardiopulmonary resuscitation until EMS transport arrives.
Although many sedation and all general anesthesia providers train in advanced cardiac life support, it is essential to appreciate that the bulk of this curriculum benefits patient management during the pre-arrest and post-arrest period. The actual success of resuscitation from cardiac arrest is predicated on an effective basic life support protocol, including early defibrillation. However, once basic life support measures are in place, providers may proceed according to the American Heart Association cardiac arrest algorithm.19 An abridged version of this protocol is provided in Figure 7. Once an advanced airway is in place, chest compressions should no longer be interrupted. A single ventilation can be provided every 6 to 8 seconds while compressions continue.
SUMMARY
Appropriate preoperative assessment and optimization of patients undergoing moderate or deep sedation are essential to minimize complications that may arise intraoperatively. Furthermore, continual monitoring of cardiovascular status provides timely information to also minimize potential occurrence of adverse events. Understanding the relevant cardiovascular physiology provides the basis for the clinician to determine the most proper means of management. In turn, this allows for optimal care for patients receiving moderate or deep sedation for their dentistry. A summary of medications addressed in this article is found in Table 2.
REFERENCES
1. Becker DE, Haas DA. Management of complications during moderate and deep sedation. Part 1: respiratory considerations. Anesth Prog. 2011;58.
2. Carlson MD. Syncope. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill; 2008.
3. Deihl RR, Linden D. Images in clinical medicine: neurocardiogenic syncope. N Engl J Med. 1998;339:312.
4. Thrush DN, Downs JB. Vagotonia and cardiac arrest during spinal anesthesia. Anesthesiology.1999;91:1171-1173.
5. Sibbald WJ, Paterson NA, Holliday RL, Baskerville J. The Trendelenburg position: hemodynamic effects in hypotensive and normotensive patients. Crit Care Med. 1979;7:218-224.
6. Coonan TJ, Hope CE. Cardio-respiratory effects of change of body position. Can Anaesth Soc J. 1983;30:424-437.
7. Lawson NW, Johnson JO. Autonomic nervous system: physiology and pharmacology. In: Barash PG, Cullen BF, Stoelting RK, eds. Clinical Anesthesia. 5th ed. Philadelphia, PA: Lippincott-Raven Publishers; 2006.
8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
9. Varon J, Marik PE. The diagnosis and management of hypertensive crisis. Chest. 2000;118:214-227.
10. Gallagher EJ. Hypertensive urgencies: treating the mercury? Ann Emerg Med. 2003;41:530-531.
11. Gray RO. Hypertension. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier, 2010.
12. Papadopoulos DP, Mourouzis I, Thomopoulos C, et al. Hypertension crisis. Blood Press. 2010;19:328-336.
13. Rodriguez MA, Kumar SK, De Caro M. Hypertensive crisis. Cardiol Rev. 2010;18:102-107.
14. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131:1949-1962.
15. Jauch EC, Cucchiara B, Adeoye O, et al. Part 11: adult stroke. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S818-S828.
16. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017.
17. American Dental Association, ADA Guidelines for the Use of Sedation and General Anesthesia by Dentists. Available at: http://www.ada.org/prof/resources/positions/statements/anesthesia_guidelines.pdf. Accessed July1, 2011.
18. Becker DE. Fundamentals of ECG interpretation. Anesth Prog. 2006;53:53-64.
19. Neumar TW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729-S767.
20. Yealy DM, Delbridge TR. Dysrhythmias. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Mosby Elsevier, 2010.
21. Kloner RA. Cardiovascular effects of the 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004;110:3149-3155.
22. Ferguson JJ, Diver DJ, Boldt M, Pasternak RC. Significance of nitroglycerin-induced hypotension with inferior wall acute myocardial infarction. Am J Cardiol. 1989;64:311-314.
23. Moye S, Carney MF, Hostege C, et al. The electrocardiogram in right ventricular myocardial infarction. Am J Emerg Med. 2005;23:793-799.
24. Pollack CV Jr, Braunwald E. 2007 update to the ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med. 2008;51:591-606.
25. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S787-S817.
26. Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S685-S705.
About the Authors
Daniel E. Becker, DDS
Associate Director of Education
General Dental Practice Residency
Miami Valley Hospital
Dayton, Ohio
Daniel A. Haas, DDS, PhD, FRCD(C)
Associate Dean, Clinical Sciences
Professor and Head of Dental Anaesthesia
Faculty of Dentistry, University of Toronto
Toronto, Ontario, Canada