You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Cancer is defined as he disease caused by an uncontrolled division of abnormal cells in a part of the body,1 but for the millions of people it has touched, cancer is so much more. To say a diagnosis of cancer changes a patient's life is a profound understatement. Few experiences leave as indelible a mark on health, relationships, and quality of life. This diagnosis opens the door to an unchosen journey for a patient and his or her family: uncountable doctor visits, unknown terminology, and continual uncertainty. Often, in the midst of this crisis, oral health and dental care are overlooked�until there is a problem.
Beyond the direct effects of the disease itself, the cancer treatment itself alters the physical well-being of patients. Modern treatment regimens to combat this disease come with a host of deleterious side effects, many of which occur in the mouth. Patients actively undergoing cancer treatment present to the dental office with a unique set of medical concerns and potential complications. Immunosuppression, radiation, and cytotoxic drugs can alter the normal healing process expected after dental procedures.
The demand for dental professionals who are trained to meet the oral healthcare needs of both patients battling cancer and cancer survivors is rising. Increasingly, patients are receiving cancer care closer to home and are therefore more dependent upon local resources for all their health care needs. Currently, there are an estimated 12.6 million people living with cancer in the United States. By the year 2020, that number is expected to increase to over 18 million. In 2012 alone, an estimated 1.64 million new cases of cancer will be diagnosed in the US.2
Dental hygienists are in a unique and essential position to make a positive impact in the overall health and quality of life of patients dealing with cancer. Making a difference begins with learning about the unique oral healthcare needs of these patients and putting into practice skills that can provide comfort. As dental professionals, hygienists can and should be a part of a comprehensive care team for an ever-growing number of people facing cancer.
The Scope of Dental Oncology
Dental oncology is a focus of dentistry dedicated to meeting the specific dental and oral healthcare needs that arise as a result of the disease process of cancer and cancer therapy.3 It includes oral medicine devoted to improving the well-being and quality of life of people with cancer. Dental oncology goes beyond the scope of general treatment to include managing normal and problematic oral soft tissues, dental supporting structures and bone, and the oral side effects subsequent to cancer therapy. While the scope of dental oncology emphasizes oral cancer education and early detection, oral cancer is not the sole focus. Dental oncology encompasses issues related to all forms of cancer.
Dental oncology care ideally begins with an assessment before the commencement of cancer treatment and culminates in the formulation of a personal oral health plan. This pretreatment evaluation allows the patient to be assessed before the effects of cancer therapy have had the opportunity to influence the norm. Oral healthcare needs that can be addressed before cancer therapy begins may often eliminate or significantly diminish complications the patient may face during treatment.4 However, sometimes cancer treatment must begin immediately. When a pre-assessment cannot be made, for whatever reason, there is still much that can be done to care for the oral health needs of the patient. Dental oncology care before, during, and after cancer therapy may include prevention and treatment of mouth and throat sores, prevention and management of dry mouth and reduced salivary flow, management of oral infections and complications (Table 1), management of oral and head and neck pain, restorative and therapeutic dentistry, customized dental hygiene instruction, management of taste alteration or loss, oral cancer screening and diagnosis, and smoking cessation.5
The link between oral health and systemic health is well documented. Less well known, however, are the effects modern cancer therapies can have on the mouth, teeth, and oral environment. Modalities of cancer treatment can cause debilitating side effects that inflict pain, decrease quality of life, and even require an interruption or discontinuation of cancer care. These side effects can become so severe that they inhibit the patient's ability to eat, thus jeopardizing essential nutrition. Similarly, secondary effects can cause the patient to withdraw socially, depriving him or her of valuable personal interaction and diminishing the desire to thrive. It is, therefore, necessary to do everything possible to lessen the potential harm caused by deleterious effects.
Oral Effects of Cancer Therapy
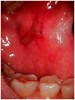
Chemotherapy employs the use of cytotoxic drugs to combat rapidly dividing cancer cells as either a primary or adjuvant modality of care. Unfortunately, most chemotherapeutic agents also affect normal rapidly dividing cells such as those of hair, bone marrow, and the lining of the gastrointestinal tract. The basement layer (stratum basale) of the oral mucosa is among those cells often affected directly by chemotherapeutic drugs. Chemotherapy also alters the vascular, inflammatory, and healing response of the oral mucosa.6 The repercussion of these changes is an ulceration that begins at the basement layer and proceeds to the surface of the mucosa causing a deep, painful sore in the mouth or oropharynx. This condition is known as mucositis (Figure 1). It is most common when the chemotherapeutic drugs are given in high doses and repeating schedules or when they are combined with radiation therapy. Clinical oral mucositis typically begins 5 to 10 days following the initiation of the regimen and lasts 7 to 14 days.7 Because the destruction of the mucosa is so deep, the ulcers can serve as conduits for infections into the bloodstream in the immunosuppressed patient. Often, mucositis can become so debilitating that it causes the patient to quit eating, which impacts systemic nutrition and compromises the patient's health. Symptoms can become so severe that it may become necessary to alter the patient's chemotherapy schedule. The severity of chemotherapy-induced mucositis is directly correlated to the degree of neutropenia, or low neutrophil count, and is most severe at the patient's nadir, the lowest point of immunosuppression.
The drugs used in chemotherapy also have a direct effect on the hematopoietic cells of the bone marrow. Patients undergoing chemotherapy may experience anemia (a decreased number of red blood cells), thrombocytopenia (a decreased number of platelets), and leukopenia (a decreased number of white blood cells). Each of these conditions is of particular interest for the dental hygienist.8
- Anemic patients may be lethargic, irritable, and forgetful. Special adjustments for the hygiene appointment might include morning appointments if the patient tires throughout the day, written home care instructions, and a blanket if the patient is cold.
- Patients with thrombocytopenia have difficulty with blood clotting. The risk for hemorrhage is confirmed with a complete blood count (CBC) and a prothrombin time and international normalized ratio (PT/INR) test. Subgingival scaling and root planing appointments should be scheduled according to the patient's thrombocytopenic status.
- Leukopenic patients, particularly those with low neutrophil counts (neutropenic), are at an increased risk for infections. This risk includes microorganisms beyond the obvious bacteria: fungal infections, especially from Candida species, and viral infections, particularly with herpes simplex virus, are more common in immunocompromised patients. Understanding the clinical presentation and symptoms of oral thrush and intraoral and perioral herpetic lesions, and being able to explain the sequella of these infections, aids in patient education.
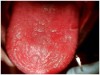
Virtually all patients undergoing chemotherapy experience, to some degree, the most common side effect: xerostomia.9 Dry mouth associated with chemotherapy can range from mild to severe. The effects of xerostomia may include alteration or reduction in taste, difficulty with chewing and swallowing, intolerance to oral medications, increased incidence of caries and oral infection, teeth sensitivity, decreased resistance to erosion, increased susceptibility to mucosal injury, and difficulty in wearing dental protheses (Figure 2). In severe instances of xerostomia, the dryness of the mouth may wake the patient at night or require water to unstick the tongue from the roof of the mouth. Patients with dry mouth may also experience dry lips or angular cheilitis. Products containing lanolin, rather than petroleum-based products, are better to treat dry lips in this situation. If angular cheilitis persists, fungi may be the cause, and the condition should be treated with topical or systemic antifungals. In the absence of saliva, its protective properties are diminished, therefore the patient may be more susceptible to intraoral and perioral infections.
A common modality of treatment for cancer, radiation therapy employs the use of ionizing radiation to destroy tumor cells. Collateral damage to the surrounding healthy tissue has decreased since the advent of intensity modulated radiation therapy (IMRT), however a history of radiation to the head and neck is still of particular concern for the dental hygienist.10 Virtually all of these patients experience severe xerostomia. Depending upon the cumulative dose of radiation, this condition may be transient or permanent. Patients experiencing xerostomia should be closely followed at least every three months until salivary flow returns to normal. Many patients undergoing head and neck radiation experience mucositis during their treatment, although the mouth sores usually resolve upon completion of the radiotherapy. Hypogeusia (diminished ability to taste, especially in a certain taste zone such as salty or sweet), dysgeusia (altered taste), and dysphagia (inability to swallow correctly) are common problems in these patients.11 Certain prophylaxis pastes may be intolerable, especially very strong flavors such as mint or cinnamon. Dental hygienists who regularly dip their hand mirrors in a mouthrinse may need to abandon that practice for these patients. Although normal taste usually returns to the patient, the process can take years, so concessions may be needed for an extended period of time.
For patients experiencing difficulty in swallowing, a routine dental cleaning can be very taxing. Constant use of a saliva ejector or repositioning into a more upright position may be necessary to keep these patients comfortable throughout the procedure. Trismus, another common side effect in patients undergoing head and neck radiation, may make the dental cleaning even more challenging for the dental hygienist and the patient. Regular at-home exercises (such as repetitive opening as wide as possible, utilizing an increasing stack of tongue depressors) or facial massage may help regain some range of motion.
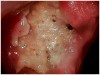
Unlike chemotherapy, some effects of head and neck radiation become more degenerative over time and continue to be an issue of concern for cancer survivors for the remainder of their lives. Chronic fatigue of the neck muscles sometimes makes it difficult to keep the head upright or move the head backwards, and care should be taken when asking the patient to reposition the head for a cleaning, radiographs, or an examination. Blockage or calcification of the carotid arteries, a long-term side effect of neck radiation, may restrict blood flow to the brain,12 although the patient may or may not exhibit symptoms of carotid artery disease. In states where dental hygienists may administer local anesthetic, the case history should be reviewed with the dentist to determine if the use of epinephrine is appropriate. In addition, because of the risk of osteonecrosis in these patients (and those with a history of intravenous bisphosphonate therapy) the dental team should educate them about the process of the condition and be alert to the signs of exposed bone (Figure 3).13
Patients who have experienced head and neck radiation are at risk for severe oral complications. Meticulous attention must be paid to oral hygiene pre-, peri-, and post-radiation, often for the remainder of the patient's life. A knowledgeable dental hygienist can be of great service to these patients in their quest for long-term oral health.
The Role of a Dental Hygienist
A dental hygienist knowledgeable in dental oncology plays several important roles throughout the patient's cancer journey. The most obvious is that of oral hygiene manager. A dental hygienist who understands how oral hygiene is related to systemic health becomes an essential team player not only in the dental oncology office, but also on the cancer care team. In this role, the hygienist can coordinate communication between the dental oncologist and the rest of the cancer care team. Hygienists can request necessary information from the physician's office and present the findings to the dental team.
Two of the most important elements are the history of present illness (HPI) and current blood work.14 The HPI helps the dentist and the dental hygienist fully understand the medical condition of the patient, including what treatment has been completed, is in progress, or is planned. It provides insight into the process of disease and any complications that have been encountered. Current blood work requested should include a complete blood count (CBC) test and a prothrombin time and international normalized ratio (PT/INR) test. Among the elements disclosed on a CBC are the absolute neutrophil count (ANC), which helps the practitioner gauge the patient's ability to combat an infection, and the platelet count, which helps determine the propensity for a bleeding episode (Table 2). If the ANC is above 2.0 (2,000/mm3), no prophylactic regimen with antibiotics is required. When the ANC is between 1.0 (1,000/mm3) and 2.0 (2,000/mm3), the patient should be premedicated with antibiotics according to the American Heart Association guidelines. Patients with an ANC of less than 1.0 (1,000/mm3) should be examined by an experienced dental oncologist or oral surgeon.
With regard to platelets, a value of 75,000 or greater does not require any platelet support therapy. A platelet range of 50,000 to 75,000 can usually be controlled with local hemostasis measures such as pressure, microfibrillar collagen, and sutures, while a value less than
50,000 indicates the necessity for a platelet transfusion one hour before the procedure and may be better handled in a location equipped to handle severely medically compromised patients.4 Values for PT/INR, partial thromoplastin time (PTT), and fibrinogen should also fall within the normal range to keep the patient in a safe zone.
Conclusion: The Cancer Care Team
The cancer care team is comprised of all caregivers working to treat the disease and improve the patient's quality of life. The leader of the cancer care team is the primary medical oncologist, to whom all other team members report. Other members of the team can include the radiation oncologist, oncological surgeon, primary care physician, other medical specialists, dental oncologist or dentist, dosimetrist, dietitian, therapists (speech, swallowing, occupational, massage, etc.), counselors, and clergy. Nurses, dental hygienists, and medical and dental assistants are invaluable in their respective areas and ensuring the entire team works as effectively as possible.
Caring for patients before, during, or after their cancer treatment is a rewarding experience. More dental hygienists knowledgeable in this area are needed to meet the unique dental and oral health care needs of patients with cancer.15 As the epidemiological forecasts continue to predict an increase in the number of new cancer cases, as well as an increase in the population of survivors, now is the time for dental hygienists to become part of the cancer care team.
References
1. Cancer. New Oxford American Dictionary. 3rd ed. New York: Oxford University Press; 2010.
2. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). National Cancer Institute. http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed August 4, 2012.
3. Abbott DM. Dental Oncology. Dallas: Dental Oncology Professionals of North Texas; 2010.
4. Pavlatos J, Gilliam KK. Oral care protocols for patients undergoing cancer therapy. Gen Dent. 2008;56(5):464-478.
5. Barker GJ, Barker BF, Grier RE. Oral Management of the Cancer Patient: A Guide for the Health Care Professional. 5th edition. Kansas City, MO: University of Missouri-Kansas School of Dentistry; 1996.
6. Raber-Durlacher JE, Barasch A, Peterson DE, Lalla RV, Schubert MM, Fibbe WE. Oral complications and management considerations in patients treated with high-dose chemotherapy. Support Cancer Ther. 2004;1(4):219-229.
7. Cheng KK. Oral mucositis, dysfunction, and distress in patients undergoing cancer therapy. J Clin Nurs. 2007; 16(11):2114- 2121.
8. Huber MA, Terezhalmy GT. The medical oncology patient. Quintessence Int. 2005;36(5):383-402.
9. National Cancer Institute. Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ�). Health Professional Version. http://www.cancer.gov/cancertopics/pdq/supportivecare/oralcomplications/HealthProfessional. Accessed August 4, 2012.
10. O'Sullivan B, Rumble RB, Warde P, Members of the IMRT Indications Expert Panel. Intensity-modulated radiotherapy in the treatment of head and neck cancer. Clin Oncol (R Coll Radiol). 2012 Jul 5. Epub ahead of print.
11. Mealey BL, Semba SE, Hallmon WW. The head and neck radiotherapy patient: Part 2. Management of oral complications. Compend Contin Educ Dent 1994;15(4):442-452.
12. Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42(9):2410-2418.
13. Estilo C, Van Poznak CH, Williams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13(8):911-920.
14. Brennan MT, Woo SB, Lockhart PB. Dental treatment planning and management in the patient who has cancer. Dent Clin North Am. 2008;52(1):19-37.
15. Bitouni A, Urankar Y. Oral health resources for cancer patients in Texas. Tex Dent J. 2012;129(5):483-488.