You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Introduction
Osteoporosis is a major public health concern that predisposes men and women to disabling and costly fractures. It is estimated that 44 million women and men aged 50 and older in the United States are at risk for these fractures.1 Osteoporotic fractures cause significant morbidity and mortality, and have a major impact on activities of daily life.
Bisphosphonates, as a class, reduce bone loss associated with diseases such as osteoporosis, Paget's disease, multiple myeloma and metastatic bone disease. By reducing bone loss, they can significantly reduce the risk of fractures.3 In the past few years, there have been case reports of osteonecrosis of the jaw (ONJ) in some patients using these agents, which has brought this issue the attention of dentists and dental hygienists. While it is important that oral care professionals be aware of ONJ, it is also important that they consider their patients' overall health status as well as their dental health.
Dentists and dental hygienists should be aware of their patients' risk of osteoporosis and fracture, so they can work with physicians and patients to weigh the risks and benefits of treatment. There are differences in ONJ incidence with low-dose oral bisphosphonate therapy compared with high-dose intravenous bisphosphonate therapy. The incidence of ONJ is very low with oral bisphosphonate use.4
Osteoporosis
Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength predisposing a person to increased risk of fracture.5
Bone tissue is constantly remodeled during a person's life by a coordinated process of resorption by osteoclasts and formation by osteoblasts. In our younger years, there is a balance between these processes so that we either build bone mass or maintain it. This compromised bone strength is due to the fact that, as individuals age, the balance of bone remodeling changes in favor of resorption. In women, this process accelerates around menopause. This imbalance between resorption and formation leads to loss of bone mass (see Figure 1), predisposing individuals to an increased risk of fracture.6 It is commonly believed that osteoporosis is an inevitable part of aging, when, in fact, the disease can be prevented and treated.7
Osteoporosis has oral health implications. Loss of teeth and ridge resorption can occur in the mouth when a person has osteoporosis.8 It is important for dental hygienists to be aware of all systemic diseases and their effects on oral health.
Prevalence, Burden of Illness and Cost
Osteoporosis represents a significant public health problem that will increase as the world population ages.9 Osteoporosis and low bone mass affect 44 million women and men aged 50 and older in the United States. The 10 million people with osteoporosis and 34 million with low bone mass represent 55 percent of the people aged 50 and older. According to NOF, by the year 2010, it is estimated that over 52 million women and men in this same age category will either have osteoporosis or be at increased risk due to low bone mass. By the year 2020, NOF expects this number to increase to over 61 million. In the U.S. alone, osteoporosis causes 1.5 million fractures annually (Figure 2). These include 300,000 hip fractures, 250,000 wrist fractures, 700,000 vertebral fractures and 300,000 fractures at other sites (Figure 3). A woman's risk of hip fracture is equivalent to her combined risk of developing breast, uterine and ovarian cancer. Half of women and a quarter of men over the age of 50 will have an osteoporotic fracture before they die.9
Osteoporosis can have a serious impact on those affected individuals. The morbidity rate for one year in those who suffer an osteoporosis-related hip fracture is up to 22 percent (Figure 4).10 Some patients are unlikely to regain pre-fracture levels of mobility and independence. Approximately 50 percent of these are still unable to walk independently one year after their hip fracture, and 70 percent have difficulty with activities of daily living (e.g., dressing, personal hygiene, food preparation).11-13
Vertebral fractures also have significant complications. Vertebral fractures can result in back pain, height loss and kyphosis,11 and result in morbidity and mortality rates similar to hip fractures (Figure 4).13
Osteoporosis is a costly disease. It has been estimated that in the U.S. in 2005, there were more than 2 million osteoporotic fractures, resulting in direct costs (e.g., hospital and outpatient costs) of $16.9 billion. Nonvertebral fractures, which occur at skeletal locations other than the spine, accounted for 73 percent of fractures and 94 percent of costs, with hip fractures in particular accounting for 14 percent of fractures and 72 percent of costs.14 Annual fractures and costs are projected to grow to more than 3 million fractures, costing $25.3 billion in direct costs by 2025.14 In addition to direct costs, indirect costs are substantial and include lost productivity, which can impact patients and family caregivers.
Risk Factors
Factors that increase the likelihood of developing osteoporosis and fractures include a personal history of fracture after age 50, low body weight, current low bone mass, history of fracture in a first-degree relative (mother, sister etc.), female gender, being thin and/or having a small frame, advanced age, a family history of osteoporosis, menopause (especially early or surgically induced), Vitamin D deficiency, use of certain medications (corticosteroids, chemotherapy, anticonvulsants and others), low testosterone levels in men, cigarette smoking, excessive use of alcohol, or being Caucasian or Asian, although African Americans and Hispanic Americans are at significant risk as well.15
Women can lose up to 20 percent of their bone mass in the five to seven years following menopause, making them more susceptible to osteoporosis.15
Treatments for Osteoporosis
The goal in treating osteoporosis is preventing fractures. The drugs approved for treatment of osteoporosis have all been proven to reduce the risk of vertebral fractures. There is varying evidence on nonvertebral (usual sites include hip, wrist, clavicle, humerus, leg and pelvis) fracture protection as well as the speed at which these drugs reduce fracture risk.
There are four types of drugs presently approved for the treatment of osteoporosis: bisphosphonates, selective estrogen receptor modulators (SERMs), calcitonin and parathyroid hormone. The first three are anti-resorptive drugs; they affect the osteoclast function in resorbing the bone. Parathyroid hormone is an anabolic drug that builds bone.
Oral Bisphosphonates
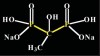
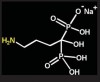
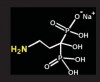
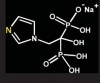
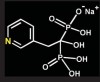
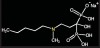
Bisphosphonates are stable analogs of pyrophosphates, which are naturally occurring modulators of bone metabolism.16 The chemical structure of bisphosphonates includes a P-C-P backbone that bestows a strong affinity for bone mineral and provides potent inhibition of bone turnover both in vivo and in vitro.17 Different bisphosphonates were developed by modifying the side chains, R1 and R2.
There are bisphosphonates that do not contain nitrogen in the R2 side chain (e.g., etidronate [Didronel®; Procter & Gamble Pharmaceuticals, Cincinnati, OH], tiludronate [Skelid®; sanofi-aventis, Paris France], and clodronate [Bonefos®; Bayer Schering Pharma, Berlin, Germany]).
Those that contain nitrogen include alendronate [Fosamax®; Merck Pharmaceuticals, Whitehouse Station, NJ], pamidronate [Aredia®; Novartis Pharmaceuticals, East Hanover, NJ], zoledronic acid [Zometa®, Novartis Pharmaceuticals], ibandronate [Boniva®; Roche Pharmaceuticals, Nutley, NJ], and risedronate [Actonel®; Procter & Gamble Pharmaceuticals]).
Bisphosphonates inhibit bone resorption by reducing osteoclastic bone resorption. Nitrogen-containing bisphosphonates are not systemically metabolized and are poorly absorbed with oral administration. Of the small amount that is absorbed (.06%), 50% is excreted unchanged in the urine, and the rest remains bound to bone, acting locally and then slowly released over time.
Differences between the bisphosphonates can be seen in the laboratory and in clinical practice. Each bisphosphonate has a unique profile of potency and binding to the bone.18
Benefits of Osteoporosis Treatment with Oral Bisphosphonates
The oral bisphosphonates currently approved by the U.S. Food and Drug Administration (FDA) for the prevention and treatment of osteoporosis are alendronate, ibandronate and risedronate. These three are the most widely prescribed therapies for osteoporosis. Data from randomized, placebo-controlled clinical trials indicate that all three of these agents reduce the risk of vertebral fractures, and that alendronate and risedronate reduce the risk of nonvertebral fractures.19-25
Side Effects of Oral Bisphosphonates
The main short-term side effects associated with the use of nitrogen-containing oral bisphosphonates are gastrointestinal (GI). In prescribing information for all oral bisphosphonates, there are precautions for GI side effects, musculoskeletal pain and ONJ. Concerns have been expressed about the long-term safety of bisphosphonates due to their long half-life, prolonged reduction of bone turnover, and the potential for reduced bone quality and strength.26 Long-term studies have followed patients treated with risedronate for up to 7 years and alendronate for up to 10 years.27,28 These studies suggest that prolonged treatment does not result in any loss of benefit or untoward side effects.
From the Actonel™ PI
Osteonecrosis, primarily in the jaw, has been reported in patients treated with bisphosphonates. Most cases have been in cancer patients undergoing dental procedures such as tooth extraction, but some have occurred in patients with postmenopausal osteoporosis or other diagnoses. Most reported cases have been in patients treated with bisphosphonates intravenously but some have been in patients treated orally.
For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment, prior to the procedure, reduces the risk of osteonecrosis of the jaw. Clinical judgment should guide the management plan of each patient based on individual benefit/risk assessment.
Osteonecrosis of the Jaw
History
Reports of ONJ first appeared in the literature in 2003 in letters to the editor and case reports related to the use of IV pamidronate and/or zoledronic acid for the management of oncological conditions: most commonly, multiple myeloma and metastatic breast cancer.29-31 Further concerns about ONJ were raised in a retrospective chart review of 63 cases of ONJ among patients receiving intravenous bisphosphonates for the management of metastatic malignancies or oral bisphosphonates for osteoporosis.2 Following these reports, FDA released a class precaution for all bisphosphonates, first IV formulations and then orals.32
A systematic review of the scientific literature in 2006 revealed that there were 368 reported cases of ONJ subsequent to the use of bisphosphonates.4 This review provided insight into the location of lesions, underlying diagnoses, type of bisphosphonate therapy and clinical observations associated with the phenomenon of ONJ. Most lesions affected the mandible and were mainly located on the posterior lingual surface near the mylohyoid ridge. Nearly a third of cases were painless, and most were preceded by tooth extraction or another invasive dental procedure. Slightly more women than men were affected (with ratio of 3:2).4 These findings indicated that patients with multiple myeloma or breast cancer with bone metastases given intravenous pamidronate or zoledronic acid were at greatest risk for ONJ; these patients accounted for 94 percent of published cases. ONJ associated with the use of oral bisphosphonate use for osteoporosis was reported in 4 percent (15/368) of the cases.4
Since this time, there have been other literature reports, case reviews and position papers published.
Clinical Characteristics of ONJ
There is currently no universally accepted definition of ONJ. The manifestations are similar to those of osteoradionecrosis (ORN) – one key difference being that involvement of the mandible is extremely rare with ORN but occurs more commonly in ONJ.4,33 The clinical signs include failure of the bone and oral mucosa to heal over six to eight weeks, jaw pain or numbness, soft-tissue swelling and infection, loose teeth and exposed bone in the oral cavity. Development of a clear understanding of ONJ is further complicated by the fact that affected patients often have serious comorbidities (e.g., advanced malignancies, coagulopathies and diabetes mellitus) for which they may be receiving chemotherapy, corticosteroids or immunosuppressive agents.
Although there is a growing concern about ONJ among patients and health care professionals, the findings should be kept in perspective. The incidence of ONJ is different in patients receiving oral bisphosphonates for osteoporosis compared with patients receiving high doses of intravenous bisphosphonates for management of malignancy.34
The risk with oral bisphosphonate therapy for osteoporosis is estimated between 1 in 10,000 and less than 1 in 100,000 patient-treatment years, whereas the estimated incidence of ONJ in patients receiving IV bisphosphonates with malignancy appears to range between 1 percent and 10 percent.34
Prevention and Treatment of ONJ
The American Dental Association (ADA), the American Association of Oral and Maxillofacial Surgeons (AAOMS), the American Society for Bone and Mineral Research (ASBMR) and the European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) have all developed positions on the prevention, recognition and management of ONJ.35-38 The lack of studies on the prevention and treatment of ONJ, however, means that current recommendations are based on clinical experience and the association's opinion rather than evidence from prospective trials.39 These position papers are summarized in Table I.
As the treatment for ONJ is limited, prevention remains important.35,36 In patients taking or considering bisphosphonate therapy, the approach to preventing ONJ is largely determined by the type of bisphosphonate therapy. Given the apparently low risk of ONJ among patients receiving oral bisphosphonates for osteoporosis, some initial guidelines have suggested that what is needed is maintenance of good oral hygiene and the same level of dental care recommended for the general population.35-38
Some health care professionals recommend dental evaluation before initiation of bisphosphonate therapy, including examination of dentures to ensure proper fit.35,36 There are no data available to suggest whether discontinuation of bisphosphonate treatment, prior to the procedure, reduces the risk of osteonecrosis of the jaw (see box).
The dentist, dental hygienist and physician should work together with the patient to discuss the benefits and risks of bisphosphonate therapy for osteoporosis and develop a treatment plan.
Summary
Osteoporosis is a major health problem that causes significant morbidity and mortality. There are a great number of men and women at risk for this disease and its complications today, and it is estimated that there will be many more in the future. Dental hygienists should be aware of the risks and benefits of treating osteoporosis, and be prepared to explain for patients the skeletal effects—including the oral health effects—of the disease. They might also encourage patients to consult their physicians regarding the research supporting the effectiveness of bisphosphonate therapy, as well as the research linking it to ONJ under certain limited conditions. Dental hygienists are in the best position to advocate optimal preventive care both in the dental office and at home to preserve the oral health of all patients including those with and at risk for osteoporosis.
Treatment is essential to reduce the risk of osteoporotic fractures. Oral bisphosphonates are an important treatment option for patients with this condition. Post-marketing case reports of ONJ in individuals taking oral bisphosphonates are rare, and this risk should be balanced against the benefits of osteoporosis treatment. Patients should be encouraged to work with their dentist, dental hygienist and physician to identify the best treatment regimen for them.
References
1. National Osteoporosis Foundation. Available at www.nof.org/.
2. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62: 527-34.
3. Bilezikian JP. Osteonecrosis of the jaw–do bisphosphonates pose a risk? N Engl J Med 2006; 355: 2278-81.
4. Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144: 753-61.
5. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. J Am Med Assoc 2001; 285: 785-95.
6. Miller RG. Osteoporosis in postmenopausal women. Therapy options across a wide range of risk for fracture. Geriatrics 2006; 61: 24-30.
7. Jeffcoat MK, Chesnut CH 3rd. Systemic osteoporosis and oral bone loss: evidence shows increased risk factors. J Am Dent Assoc 1993; 124(11): 49-56.
8. Melton LJ 3rd. Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 2003;18:1139-41.
9. National Osteoporosis Foundation. Fast facts. Available at http://www.nof.org/aboutosteoporosis/bonebasics/top10myths.
10. Johnell O, Kanis JA, Odén A et al. Mortality after osteoporotic fractures. Osteoporos Int 2004; 15: 38-42.
11. Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med 1997; 103(2A):12S-19S.
12. Koot VC, Peeters PH, de Jong JR et al. Functional results after treatment of hip fracture: a multicentre, prospective study in 215 patients. Eur J Surg 2000; 166: 480-5.
13. Pasco JA, Sanders KM, Hoekstra FM et al. The human cost of fracture. Osteoporos Int 2005; 16: 2046-52.
14. Burge R, Dawson-Hughs B, Solomon D, et al. Incidence and economic burden of osteoporosis related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22: 465-75.
15. National Osteoporosis Foundation. Fast facts. Available at: www.nof.org/aboutosteoporosis/bonebasics/riskfactors.
16. Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci 2006; 1068: 367-401.
17. Nancollas GH, Tang R, Phipps RJ et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006; 38: 617-27.
18. RGG Russell. Bisphosphonates an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 1007; 1117: 209–57. C_ 2007 New York Academy of Sciences.
19. Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535-41.
20. Black DM, Thompson DE, Bauer DC et al. Fracture Intervention Trial. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85: 4118-24.
21. Harris ST, Watts NB, Genant HKet al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. J Am Med Assoc 1999;282: 1344-52.
22. McClung MR, Geusens P, Miller PD et al.; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001;344: 333-40.
23. Chesnut CH 3rd, Skag A, Christiansen C et al.; for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19: 1241-9.
24. Cummings SR, Black DM, Thompson DE et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. J Am Med Assoc 1998; 280:2077-82.
25. Reginster J, Minne HW, Sorensen OH et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11: 83-91.
26. Liberman UA. Long-term safety of bisphosphonate therapy for osteoporosis: a review of the evidence. Drugs Aging 2006; 23: 289-98.
27. Mellstrom DD, Sorensen OH, Goemaere S et al. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462-8.
28. Bone HG, Hosking D, Devogelaer JP et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350: 1189-99.
29. Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. [Letter] J Clin Oncol 2003; 21: 4253-54.
30. Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg 2003; 61: 1104-7.
31. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115-7.
32. MedWatch – Safety Information. Available at www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150837.htm. Accessed Mar 12, 2007.
33. Badros A, Weikel D, Salama A et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006; 24: 945-52.
34. American Association of Oral and Maxillofacial Surgeons (AAOMS). Position paper on bisphosphonate-related osteonecrosis of the jaws. 2006. Available at www.aaoms.org/docs/position_papers/osteonecrosis.pdf.
35. American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137: 1144-50.
36. AAOMS position paper on bisphosphonate-related osteonecrosis of the Jaws. J Oral Maxillofac Surg 2007;65; 369-76.
37. ASBMR Task Force on Bisphosphonate-Associated ONJ. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007;22(10):1479-91.
38. Rizzolli R. Impact of osteonecrosis of the jaw on osteoporosis management: executive summary of an ESCEO and Foundation for Research on Osteoporosis and other Bone disease working group meeting. Aging Health 2007 3(6) e-pub. Expert panel recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaws. June 2004.
39. Shane E, Goldring S, Christakos S et al. Osteonecrosis of the jaw: more research needed. J Bone Miner Res 2006;21: 1503-05.
Additional Resources
Silverman SL. Quality-of-life issues in osteoporosis. Curr Rheumatol Rep 2005; 7:39-45.
Boonen S, Autier P, Barette M et al. Functional outcome and quality of life following hip fracture in elderly women: a prospective controlled study. Osteoporos Int 2004; 15: 87-95.
Hallberg I, Rosenqvist AM, Kartous L et al. Health-related quality of life after osteoporotic fracture. Osteoporos Int 2004;15: 834-41.
Migliorati CA, Casiglia J, Epstein J et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc 2005; 136: 1658-68.
About the Authors
Jon B. Suzuki, DDS, PhD, MBA, has a Presidential appointment as professor of microbiology in the School of Medicine and professor of periodontology in the School of Dentistry at Temple University, Philadelphia, Penn., where he also serves as associate dean for graduate education, research and international affairs as well as director of graduate periodontology. He has served as dean of the School of Dental Medicine and chief of hospital dentistry at the University of Pittsburgh. He received his DDS from Loyola University of Chicago, PhD in microbiology from the Illinois Institute of Technology, and MBA from the Katz Graduate School of Business of the University of Pittsburgh. He is on the faculty of the U.S. Navy National Naval Medical Command, Bethesda, Md., and also holds professorships at several universities here and abroad. He has served in leadership roles for numerous professional and academic societies and government entities, and he has received many honors from academic and professional institutions. He is in private practice limited to periodontics in Philadelphia. His many publications include the textbook on medical technology.
Andrea B. Klemes, DO, FACE, is the North American medical director for Procter & Gamble Pharmaceuticals, in the musculoskeletal category. She is a board-certified internist and endocrinologist. She completed her medical residency at Cabrini Medical Center in New York City, and her endocrine fellowship at the Medical College of Georgia. She was in private endocrine practice for 10 years in Tallahassee, Fla. before coming to work for Procter & Gamble.