You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The goal of periodontal therapy is the maintenance of the dentition and/or its implant replacements in a state of health, comfort, function, and esthetics for the duration of a patient’s life. With the increasing number of patients receiving dental implants and the aging of existing dental implants in service, the prevalence of peri-implant inflammatory problems presenting to clinical practices is rising.1
As well-stated by Newman et al: “The periodontal care of the public is primarily the concern of the general dentist, who cannot disregard his or her responsibility to examine, treat, or refer all periodontal problems. The high incidence of periodontal problems and the close relationship between periodontal and restorative dental therapies make this an incontrovertible point.”2 The maintenance phase of treatment most often is conducted in the general practice and, therefore, it is critical for general dentists to understand the importance of monitoring and maintaining not only periodontal health but also peri-implant health.3,4
A clear understanding of the signs of disease recurrence is crucial so that early and definitive action can be taken to prevent further clinical attachment and bone loss around teeth and/or implants, which might otherwise go unnoticed until advanced stages. This article provides a review of periodontal maintenance goals, procedures, and efficacy involving natural teeth and dental implants, as well as makes recommendations on when and how to address signs of recurrent disease activity during periodontal maintenance (PM).
Goals of Periodontal and Peri-implant Maintenance
The primary etiology of periodontal and peri-implant diseases is pathogenic bacterial plaque in a susceptible patient. Because patient susceptibility currently cannot be altered predictably, PM is aimed at minimizing bacterial plaque to limit the risk of further disease activity that causes continued periodontal and peri-implant attachment and bone loss. This therapy usually is delivered at 3-month intervals, but the specific interval is tailored to each patient’s presentation and needs. PM also allows for close monitoring of the patient so that if periodontal or peri-implant disease recurrence or other oral health problems should occur, early intervention can be provided.
The goals of PM are:5
- to minimize the recurrence and progression of periodontal diseases in patients who have been treated previously for gingivitis and periodontitis.
- to reduce the incidence of tooth loss by monitoring the dentition and any prosthetic replacements of the natural teeth (including dental implants).
- to increase the probability of locating and treating, in a timely manner, other diseases or conditions found within the oral cavity.
Maintenance and Periodontitis
Periodontitis is a chronic inflammatory disease of natural teeth that requires lifelong management. Periodontitis presents in several forms, all of which involve loss of supporting bone and periodontal ligament. Long-term studies on the success of periodontal therapy demonstrate that a well-planned and executed periodontal treatment plan (including long-term maintenance) is effective in controlling disease progression and preserving the dentition in most patients.6,7
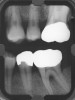
After complete management of moderate to severe periodontitis, including scaling and root planing and in some cases periodontal surgery, subsequent regular periodontal maintenance is reported to prevent tooth loss in up to 85% of patients over a long-term follow-up interval of up to 53 years.6,8 This high success rate of periodontal treatment is comparable to that of dental implants in replacing teeth lost to severe periodontal diseases, highlighting the predictability of maintaining the natural dentition in most patients with periodontal diseases9,10 (Figure 1A and Figure 1B).
It is critical to understand, however, that when periodontal treatment is delivered without a tightly controlled lifelong PM regimen, the benefits of therapy often are lost, allowing the recurrence of disease activity, further loss of periodontal tissues, and tooth mortality.8,11 Long-term outcome studies on the delivery of periodontal treatment without any posttreatment PM have found that unmaintained patients lose two to three times the number of teeth and have a twofold increased risk for requiring re-treatment in the form of scaling and root planing or periodontal surgery.8,12
This supports an evidence-based belief that periodontal treatment, in the form of surgical or nonsurgical modalities, is ineffective in the long term without an effective PM regimen. Furthermore, increased emphasis has been placed on the control of periodontal inflammation recently, with the discovery of its role as a risk factor for several chronic systemic diseases. Long-term PM provides an opportunity to control systemic low-grade periodontally derived inflammation in a lifelong treatment model.13
Maintenance and Peri-Implant Disease
The success of endosseous dental implants has been attributed to their functional ankylosis or bone anchorage, termed osseointegration.14 While dental implants are a highly successful treatment modality, they are susceptible to periodontal pathogens and a stimulated host inflammatory reaction, which may lead to peri-implant soft-tissue inflammation and bone loss.15
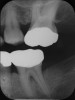
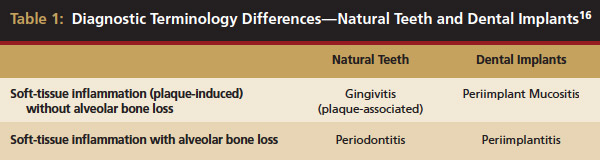
Inflammation limited to soft tissues around a dental implant may result from dental plaque colonization and is termed peri-implant mucositis. This is a reversible inflammatory condition limited to the soft tissues around the implant (without any bone loss), analogous to gingivitis around natural teeth. A diagnosis of peri-implantitis results when the inflammation spreads apically, causing progressive loss of osseointegrated supporting bone, analogous to periodontitis around natural teeth16 (Figure 2A and Figure 2B). It is essential for practitioners to be familiar with these diagnostic terms when assessing the long-term success of implants and peri-implant health (Table 1).
 |
It is also important that the etiology and chronology of bone loss around implants be identified. Peri-implant bone loss resulting from surgical trauma or technique, such as pressure necrosis from inadequate osteotomy preparation or coronal bony voids from excessive countersinking, must be differentiated from peri-implant bone loss resultant from bacterial plaque-mediated immunoinflammatory bone loss. Implants placed using a subcrestal platform position tend to have deeper baseline probing depths than those placed supracrestally; therefore, it is important to know the baseline probing depth after initial healing to allow monitoring for changes over time.
The incidence and prevalence of peri-implantitis has been reported in the literature. Berglundh et al1 found the incidence of peri-implantitis was up to 14.4% and appeared to be related to the number of years that the fixtures were in service. Additionally, Roos-Jansåker et al17 reported that of implant cases not enrolled in a regular posttreatment PM program, 16% demonstrated peri-implantitis by 7 to 9 years after implant placement. The incidence of peri-implantitis may be underestimated because few studies exist with follow-up longer than 10 years. Considering that chronic periodontitis often takes 30 years or more to develop, the current literature does not evaluate long-term peri-implantitis incidence sufficiently.
While dental implants have been shown to be successful in patients with severe periodontitis, several researchers have demonstrated the bacterial profile around implants is similar to the patient’s natural teeth.18,19 Dental implants may harbor a complex microbiota with a large proportion of known periodontal pathogens, such as Porphymonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum, which have been associated with the onset of peri-implant mucositis and peri-implantitis.20-22
Additionally, studies examining long-term follow-up dental implants in patients with a history of periodontitis have suggested a higher incidence of soft-tissue inflammation (mucositis) and peri-implantitis, as well as a slightly higher failure rate.23
These findings support the recommendation that patients with dental implants require regular and careful evaluation at selected PM intervals to detect any clinical signs and symptoms of peri-implant disease.
Types of Periodontal Maintenance
PM programs may be prescribed for several scenarios. Patients may enter PM for reduction of disease risk, after completion of nonsurgical or surgical therapy, or for reduction of disease progression when definitive treatment cannot be rendered. For this reason, PM has been divided into four types24 (Table 2). Selection of the appropriate type of maintenance for each patient allows the therapist to approach every maintenance visit with the appropriate mindset and also gives an idea of the case’s direction as a communication tool between patients and providers.
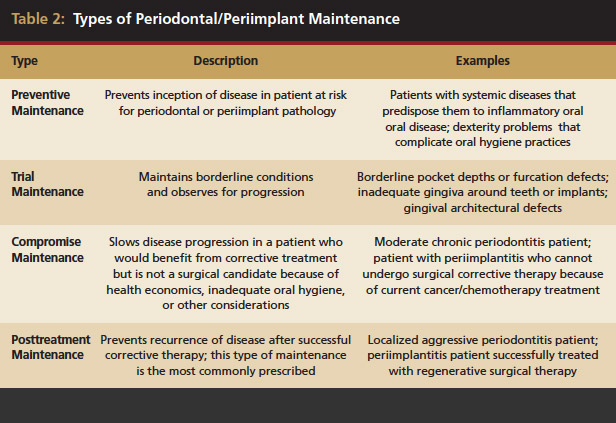 |
- Preventive PM: Intended to prevent inception of disease in those who currently do not have periodontal pathology (eg, patients at high risk for development of periodontal or peri-implant problems because of systemic disease or dexterity problems that prevent practicing hygiene).
- Trial PM: Intended to maintain borderline periodontal conditions to assess over time any progression of disease and the need for further treatment (eg, borderline pocket depths or furcation defects, inadequate gingiva around teeth or implants, or gingival architectural defects).
- Compromise PM: Intended to slow disease progression in patients who would benefit from corrective treatment but are not surgical candidates because of health, economics, inadequate oral hygiene, or other considerations. This type also includes situations in which periodontal or peri-implant defects persist after corrective therapy attempts (eg, patients with moderate chronic periodontitis or peri-implantitis who cannot undergo treatment because of current gastric cancer treatment).
- Posttreatment PM: Designed to prevent recurrence of disease after successful corrective therapy. This type of PM is the most commonly prescribed (eg, patients with localized aggressive periodontitis or peri-implantitis who have been treated successfully with regenerative surgical therapy).
Frequency of Periodontal Maintenance
Soon after the bacterial oral biofilm is disturbed by plaque removal, it begins to reform. Eventually, the plaque becomes increasingly complex and gingival inflammation emerges.25 Bacterial repopulation to pretreatment levels after scaling and root planing has been studied and, while it is variable between patients, may be seen as early as 42 days.26 However, in most patients, the return to near-baseline bacterial levels occurs within 3 to 6 months.27
Based on these studies, an initial PM interval of 3 months is selected for susceptible patients. While a 3-month interval is appropriate for many patients, PM needs to be tailored to each patient’s level of disease activity and his or her ability and willingness to perform adequate oral hygiene.
Patients who demonstrate persistent gingival inflammation, increasing probing depths, calculus formation, and poor plaque control around natural teeth or dental implants may require more frequent PM visits (ie, every 2 months instead of every 3 months). Conversely, for patients who demonstrate excellent plaque control, healthy gingiva, and stable probing depths, lengthening the PM interval may be appropriate (ie, every 4 months instead of every 3 months).
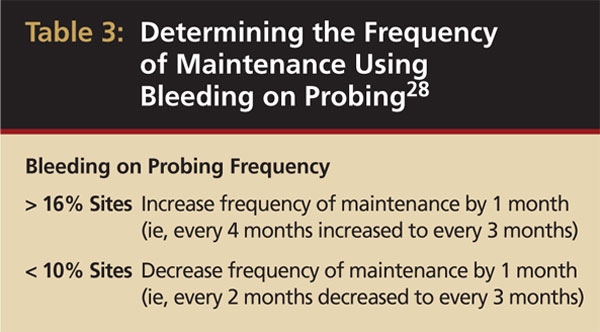
Bleeding on probing has been proposed as one method for determining the PM interval. One study followed patients with treated chronic periodontitis who were on PM for 4 years.28 It was found that bleeding on probing at more than 16% of sites was associated with increased attachment loss, while bleeding on probing at < 10% of sites was not. Based on these findings, the researchers suggested the PM interval can be shortened or lengthened, depending on the percentage of bleeding sites found at each PM visit (Table 3). However, because bacterial repopulation occurs by 6 months in all patients, this interval should not exceed 6 months. Around dental implants, it appears that bleeding on probing provides similar predictability as an indicator of disease as with natural teeth.29 These findings facilitate an evidence-based approach for clinicians to determine recall intervals for PM patients around both natural teeth and dental implants.
 |
Maintenance Visits
PM visits should include an update of the medical and dental history; thorough extraoral and intraoral examinations that include oral cancer screening; periodontal and implant reevaluation; radiographic review; check of occlusion; removal of supra- and subgingival bacterial plaque and calculus; selective root planing; implant debridement if indicated; polishing if necessary; and a review of the patient’s oral hygiene efficacy.5,24
A typical appointment should take no more than 60 minutes, with at least 25% of this time devoted to mechanical debridement.24 If signs of persistent disease activity are evident at the PM visit, such as persistent gingival or peri-implant inflammation, bleeding on probing, implant mobility, and/or increasing probing depths, steps should be taken to address these findings to maintain health.
If active treatment is performed during maintenance, a subsequent reevaluation should be scheduled at a 4- to 6-week interval to ensure any intervention was successful in controlling periodontal or peri-implant diseases and whether further treatment is necessary.30 This interval is recommended based on classic studies by Proye et al as well as Morrison et al, which have demonstrated posttreatment periodontal healing (around natural teeth) requires at least 4 weeks for reductions in pocket depth and gains in clinical attachment levels to fully occur.30,31 There appears to be a paucity of specific studies on similar posttreatment response around ailing dental implants. However, most current studies suggest peri-implant wound-healing processes are similar to natural teeth, and therefore a similar interval of 4 to 6 weeks is empirically suggested for reevaluation after treatment of inflammation around dental implants.16,29
Management of Recurrent Disease During Maintenance: Natural Teeth
Signs of disease activity around natural teeth are indicated by the presence of bleeding on probing, visible soft-tissue inflammation, increasing probing depth, suppuration, radiographic bone loss, and/or increasing mobility that presents during maintenance. A combination of bleeding on probing at successive PM visits and increasing probing depth of > 1 mm has been found to be 87% predictive of periodontal bone and attachment loss.32
When such findings are encountered during PM, early action should be taken to ensure management. The first step in addressing disease activity is local debridement of the site. For natural teeth, this involves high-quality scaling and root planing, using a combination of ultrasonic instruments and sharp hand curettes to ensure effective removal of plaque and calculus. Human clinical studies have demonstrated ultrasonic instruments have a tremendous advantage over hand instrumentation alone for several reasons. First, accessing the challenging anatomic features of multirooted teeth with furcation involvement is better achieved with ultrasonics.33-35 Second, ultrasonics have been found to be more efficient and equally effective compared to hand instruments when applied to single rooted teeth.33,36 Third, a combination of sonic and hand curette instruments for nonsurgical therapy has been shown to be more effective in calculus removal than either modality used alone.37 Often, a single intervention of nonsurgical therapy is successful in controlling disease activity during the PM phase. Long-term studies have shown that after periodontal surgery, recurrent disease developing during PM responds favorably to nonsurgical therapy in 88% of cases.38
The use of adjunctive treatments, such as introduction of additional oral hygiene aids, use of locally delivered antibiotics (such as minocycline microspheres [Arestin®, OraPharma Inc, Warminster, PA]), host modulation therapy (low-dose doxycycline [Periostat]), subgingival irrigation, or prescription mouth rinses may be helpful in controlling signs of persistent disease.
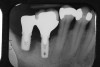
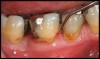
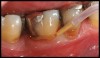
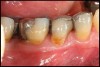
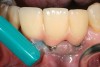
Locally delivered, controlled-release agents are a popular treatment adjunct and serve to augment scaling and root planing to help improve outcomes. Clinical studies have shown that use of such agents can provide an average of 0.5-mm additional pocket depth reduction over scaling and root planing alone, so the practitioner must weigh the cost-benefit ratio of using these products in each patient situation.39,40 A guideline for the appropriate use of locally delivered antimicrobials has been provided by the American Academy of Periodontology.41 These materials are contraindicated in patients with (1) multiple 5-mm pocket depths in one quadrant; (2) anatomic defects caused by periodontal diseases (intrabony defects); and (3) when the previous use of locally delivered agents has failed to control disease. As noted before, a follow-up reevaluation always should be completed to evaluate the success of any therapeutic intervention, generally at 4 to 6 weeks after treatment has been rendered30,31 (Figure 3A through Figure 3D).
If adjunctive treatments have been unsuccessful in controlling signs of disease, providers should re-treat the patient within their clinical competency and experience level or refer the patient to a specialist in periodontics for comanagement of the case. For recommendations on risk assessment and when to refer a patient to a periodontist, it is suggested that practitioners refer to the 2006 American Academy of Periodontology position paper, entitled "Guidelines for the Management of Patients with Periodontal Diseases", which is at http://www.perio.org/resources-products/pdf/management.pdf.42 These guidelines, while initially controversial, are intended to assist the general practitioner in quickly identifying only patients who are at the greatest risk and most appropriately suited for specialty care. These guidelines are not intended to serve as a medico-legal standard of care and do not replace the knowledge, skill, and abilities of the practitioner.42 Ultimately, it is the patient who will benefit from thorough risk assessment and appropriate source of care.
Management of Recurrent Disease during Maintenance: Dental Implants
Signs of inflammatory disease activity around dental implants appear to be similar to those noted above for natural teeth, including bleeding on probing, visual inflammation, radiographic bone loss, and implant mobility.29
Following implant restoration, patients should be reevaluated regularly (ie, every 3 to 4 months) during the first year. After the first year, the peri-implant-tissue response should be evaluated and then the required customized frequency of PM should be determined, as discussed earlier in this article. Initial bone loss during the first year can be expected to be near the level of the first thread or may be less in systems that include platform switching.43 Additional bone loss of approximately 0.1 mm per year for the first 5 years (up to a total of 1.5 mm) is considered normal.44 Complete seating of the associated parts (abutment and/or restoration), occlusal overloading, absence of restorative overhangs, and the removal of all restorative cements also should be verified from the radiograph because these problems can predispose to long-term complications.45
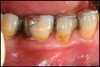
Conventional periodontal therapy should be instituted if inflammation develops around an implant. Therapy should include efforts to improve patient oral hygiene, using similar methods as around natural teeth (Figure 4A and Figure 4B). Lang et al suggested a novel, systematic stepwise approach for the prevention and treatment of peri-implant diseases referred to as the cumulative interceptive supportive therapy (CIST) protocol46 (Table 4). This system is based on periodic monitoring with implementation of treatment as thresholds for a particular condition are met. The first step is protocol A, then B and, if conditions continue to worsen, the case may require more advanced treatment, which may include comanagement with a specialist who has implant training to execute protocol C, and finally D.20 Protocol A is used to control inflammation in peri-implant mucositis, that is, implants with minimal increase in pocket depth, slight (+) bleeding on probing, marginal erythema, plaque, and/or calculus. The therapeutic endpoint is to resolve inflammation with cautious mechanical debridement (using plastic curettes and rubber cup prophylaxis), twice-daily swabbing with 0.12% chlorhexidine, and a review of home care and patient motivation. Protocol B is initiated for conditions that exhibit similar mucositis features but with deeper pocket depths (4 mm to 5 mm); however, there is still no loss of supporting bone. The treatment should include the therapies of protocol (A), plus locally delivered antibiotic (minocycline microspheres, doxycycline gel) at the infected implant site(s). Recent studies have shown the use of minocycline microspheres may be beneficial in treatment of peri-implant mucositis and peri-implantitis.46 Management of early peri-implantitis, protocol C, requires a more robust approach and is used in conditions with evidence of osseointegrated bone loss of < 2 mm and pocket depths > 5 mm. The strategy should comprise the modalities for protocols A and B with the addition of systemic antibiotic therapy (metronidazole 250 mg t.i.d. for 7 days or amoxicillin 500 mg t.i.d. for 10 days).
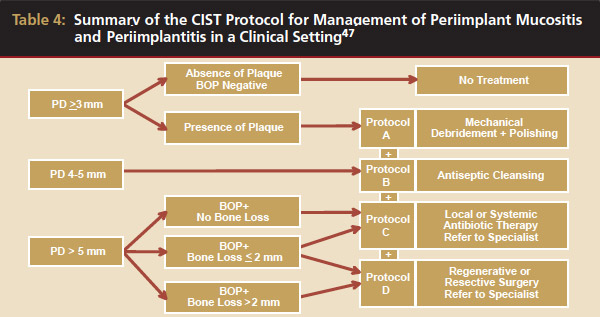
Furthermore, periodontal surgical access for surface decontamination (citric acid 1 to 2 minutes or tetracycline 250 mg, 5 mL for 5 minutes) should be considered. Protocol D is initiated in circumstances of frank peri-implantitis that reveal probing depths (> 5 mm), (+) bleeding on probing, plaque/calculus, and peri-implant bone loss of > 2 mm. This strategy requires periodontal surgical intervention for chemical disinfection, osseous resection, and/or guided bone regeneration (GBR). GBR will attempt to salvage the implant through bone regeneration techniques with the use of resorbable or nonresorbable semipermeable membranes and a bone replacement graft (such as freeze-dried bone allograft or anorganic bovine bone). In clinical practice, CIST is aimed at early detection and methodical stepwise treatment, which may rescue and reverse the fate of the ailing or failing endosseous dental implant.47
Compliance with Periodontal Maintenance
Compliance with prescribed PM is often a challenge. Wilson conducted a survey of compliance rates in a private practice setting and found only 16% of patients were totally compliant with PM, 32% of patients never returned for PM, and the remaining 52% were erratic with compliance.48 These numbers appear to be consistent with the medical literature on compliance. While the outlook on compliance may seem grim, Wilson et al found in a later study that compliance could be improved twofold by several methods, including sending reminder cards to patients and scheduling patients far in advance.49 Additionally, compliance seems to increase with growing complexity of treatment, which may result in improved PM compliance among patients who have undergone significant periodontal surgery and/or dental implant therapy.
Because compliance with PM is so critical to the long-term success of periodontal and dental implant therapy, the importance of PM must be emphasized with patients from the onset of treatment by all dental providers and at each follow-up maintenance visit. Patients must understand that the success of periodontal therapy rendered depends on compliance with PM and dental implants are not impervious to later inflammatory complications.
Maintenance and Periodontal Medicine
Recent studies support the connection of periodontal diseases to several systemic diseases and conditions through chronic inflammatory mediators.50 These include cardiovascular disease, stroke, chronic obstructive pulmonary disease, preterm low-birth-weight, and type 2 diabetes mellitus (dependent on level of blood glucose control). Many of these diseases and conditions are chronic and develop as a result of constant circulation of low-grade inflammatory cytokines and acute-phase proteins, such as interleukin-1 beta, tumor necrosis factor-alpha, and C-reactive protein, which have been shown to be increased by periodontal inflammation.51 While at first glance, it may appear that short-term periodontal treatment may affect these disease relationships, in general, it is doubtful that a single session of scaling and root planing will reverse, for example, 30 years of atherosclerosis. Therefore, the role of the dental practitioner in periodontal medicine best fits into a PM model. Lifelong PM is important not only in achieving periodontal health but also in minimizing the circulation of inflammatory cytokines for many years to mitigate the periodontally derived inflammatory risk factors for systemic diseases.
Conclusion
Periodontal treatment success, including both nonsurgical and surgical therapy, is dependent on appropriate maintenance. PM therapy also applies to dental implants, as they have been shown to be susceptible to peri-implant disease. In addition, long-term control of periodontal inflammation may reduce the risk of several systemic diseases and conditions. It is the general practitioner’s responsibility to evaluate each patient’s dental history and prescribe appropriate periodontal and peri-implant maintenance care, as well as to identify when conventional treatment is failing and to execute a prompt and appropriate solution, which includes use of adjunctive agents, surgery, or referral to a periodontist. The keys to success include consistent reminders sent to the patients on the importance of long-term maintenance in preventing periodontal or peri-implant disease progression, as well as early identification and treatment of inflammatory and biomechanical problems to minimize their impact. This will maximize the likelihood of maintenance of natural teeth and dental implants in health, comfort, function, and esthetics for the duration of the patient’s life.
Disclosure
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
1. Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29(suppl 3):197-212.
2. Newman MG, Takei HH, Carranza FA. Clinical Periodontology. 9th ed. Philadelphia, PA: Saunders; 2002.
3. Lang NP, Nyman SR. Supportive maintenance care for patients with implants and advanced restorative therapy. Periodontol 2000. 1994;4:119-126.
4. Preshaw PM, Heasman PA. Periodontal maintenance in a specialist periodontal clinic and in general dental practice. J Clin Periodontol. 2005;32(3):280-286.
5. Cohen RE, Research, Science and Therapy Committee, American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003;74(9):1395-1401.
6 Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49(5):225-237.
7. Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. 2004;31(9):749-757.
8. Becker W, Becker BE, Berg LE. Periodontal treatment without maintenance. A retrospective study in 44 patients. J Periodontol. 1984;55(9):505-509.
9. Lekholm U, Gunne J, Henry P, et al. Survival of the Brånemark implant in the partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants. 1999;14(5):639-645.
10. Testori T, Del Fabbro M, Feldman S, et al. A multicenter prospective evaluation of 2-months loaded Osseotite implants placed in the posterior jaws: 3-year follow-up results. Clin Oral Implants Res. 2002;13(2):154-161.
11. Nyman S, Lindhe J, Rosling B. Periodontal surgery in plaque-infected dentitions. J Clin Periodontol. 1977;4(4):240-249.
12. Fardal O, Linden GJ. Re-treatment profiles during long-term maintenance therapy in a periodontal practice in Norway. J Clin Periodontol. 2005;32(7):744-749.
13. Scannapieco FA. Systemic effects of periodontal diseases. Dent Clin North Am. 2005;49(3):533-550.
14. Brånemark PI. Introduction to osseointegration. In: Brånemark PI, Zarb G, Albrektsson T. Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago, IL: Quintessence; 1985:11-76.
15. Hultin M, Gustafsson A, Hallström H, et al. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res. 2002;13(4):349-358.
16. Esposito M, Hirsch J, Lekholm U, et al. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: a review of the literature. Int J Oral Maxillofac Implants. 1999;14(4):473-490.
17. Roos-Jansåker AM, Lindahl C, Renvert H, et al. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33(4):290-295.
18. Quirynen M, Listgarten MA. Distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Brånemark. Clin Oral Implants Res. 1990;1(1):8-12.
19. Mombelli A, Marxer M, Gaberthüel T, et al. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22(2):124-130.
20. Weyant RJ, Burt BA. An assessment of survival rates and within-patient clustering of failures for endosseous oral implants. J Dent Res. 1993;72(1):2-8.
21. Tonetti MS, Schmid J. Pathogenesis of implant failures. Periodontology 2000. 1994;4:127-138.
22. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4) Roos-Jansåker AM, Renvert H, Lindahl C, et al. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33(4):296-301.
24. Schallhorn RG, Snider LE. Periodontal maintenance therapy. J Am Dent Assoc. 1981;103(2):227-231.
25. Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177-187.
26. Mosquès T, Listgarten MA, Phillips RW. Effect of scaling and root planing on the composition of the human subgingival microbial flora. J Periodontal Res. 1980;15(2):144-151.
27. Slots J, Mashimo P, Levine MJ, et al. Periodontal therapy in humans. I. Microbiological and clinical effects of a single course of periodontal scaling and root planing, and of adjunctive tetracycline therapy. J Periodontol. 1979;50(10):495-509.
28. Lang NP, Joss A, Orsanic T, et al. Bleeding on probing. A predictor for the progression of periodontal disease. J Clin Periodontol. 1986;13(6):590-596.
29. Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants. 2004;19(suppl):116-127.
30. Proye M, Caton J, Polson A. Initial healing of periodontal pockets after a single episode of root planing monitored by controlled probing forces. J Periodontol. 1982;53(5):296-301.
31. Morrison EC, Ramfjord SP, Hill R. Short-term effects of initial, nonsurgical periodontal treatment (hygienic phase). J Clin Periodontol. 1980;7(3):199-211.
32. Claffey N, Egelberg J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J Clin Periodontol. 1995;22(9):690-696.
33. Matia JI, Bissada NF, Maybury JE, et al. Efficiency of scaling of the molar furcation area with and without surgical access. Int J Periodontics Restorative Dent. 1986;6(6):24-35.
34. Wylam JM, Mealey BL, Mills MP, et al. The clinical effectiveness of open versus closed scaling and root planing on multi-rooted teeth. J Periodontol. 1993;64(11):1023-1028.
35. Bower RC. Furcation morphology relative to periodontal treatment. Furcation entrance architecture. J Periodontol. 1979;50(1):23-27.
37. Gellin RG, Miller MC, Javed T, et al. The effectiveness of the Titan-S sonic scaler versus curettes in the removal of subgingival calculus. A human surgical evaluation. J Periodontol. 1986;57(11):672-680.
38. Kaldahl WB, Kalkwarf KL, Patil KD, et al. Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down. J Periodontol. 1996;67(2):103-108.
39. Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8(1):79-98.
40. Bonito AJ, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J Periodontol. 2005;76(8):1227-1236.
41. Academy Report: American Academy of Periodontology statement on local delivery of sustained or controlled release antimicrobials as adjunctive therapy in the treatment of periodontitis. J Periodontol. 2006;77(8):1458.
42. Academy Report. Guidelines for the management of patients with periodontal diseases. J Periodontol. 2006;77(9):1-4.
43. Canullo L, Rasperini G. Preservation of peri-implant soft and hard tissues using platform switching of implants placed in immediate extraction sockets: a proof-of-concept study with 12- to 36-month follow-up. Int J Oral Maxillofac Implants. 2007;22(6):995-1000.
44. Albrektsson T, Zarb G, Worthington P, et al. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
45. Lang B, Worthington P, LaVelle W. Osseointegration in Dentistry: An Introduction. Chicago, IL: Quintessence; 1994:121.
46. Salvi GE, Persson GR, Heitz-Mayfield LJ, et al. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18(3):281-285.
47. Lang NP, Berglundh T, Heitz-Mayfield LJ, et al. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxillofac Implants. 2004;19(suppl):150-154.
48. Wilson TG Jr. Compliance. A review of the literature with possible applications to periodontics. J Periodontol.1987;58(10):706-714.
49. Wilson TG Jr, Hale S, Temple R. The results of efforts to improve compliance with supportive periodontal treatment in a private practice. J Periodontol. 1993;64(4):311-314.
50. Scannapieco FA. Systemic effects of periodontal diseases. Dent Clin North Am. 2005;49(3):533-550.
51. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 suppl):2106-2115.
About the Authors
Nicholas D. Shumaker, DDS, MS, Department Head for Periodontics, Naval Health Clinic Quantico, Quantico, Virginia
Brett T. Metcalf, DDS, MS, Department Head for Periodontics, Naval Hospital Naples, Naples, Italy
Nicholas T. Toscano, DDS, MS, Department Head for Periodontics, Washington Navy Yard, Washington, DC
Dan J. Holtzclaw, DDS, MS, Department Head for Periodontics, Naval Hospital, Pensacola, Florida