You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
The biologic complications of peri-implant disease—particularly when it progresses from peri-implant mucositis to peri-implantitis—is a significant problem that threatens the survival of dental implants and possibly their status as the preferred approach to replacing a missing tooth or teeth. Periodontal disease and inadequate oral hygiene have been among the peri-implant disease risk factors specified in the Consensus Report of the Sixth European Workshop on Periodontology.1
As these two factors can be controlled with the combined the efforts of the patient and treating dental team—ie, hygienist, dentist/periodontist—the need to focus on plaque control education and more frequent maintenance and monitoring visits cannot be overstated.
Periodontal Disease vs. Peri-Implant Disease
Periodontal disease has been more precisely defined than peri-implantitis. Based on established criteria, it is estimated that periodontal disease affects as many as 47.2% of American adults, including 70.1% of adults 65 years and older.2
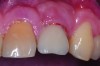
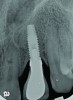
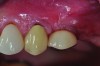
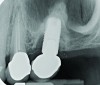
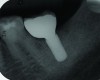
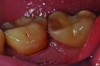
Peri-implant diseases present in two forms—peri-implant mucositis (Figure 1 and Figure 2) and peri-implantitis (Figure 3 and Figure 4). Both involve an inflammatory reaction in the tissues surrounding an implant—ie, bleeding on probing and/or suppuration; probing depths usually exceed 4 mm.1,3 However, when there is also bone loss present beyond the physiologic remodeling that may occur after implant placement, a diagnosis of peri-implantitis should be made, as this condition, when compared to mucositis, is far more serious and difficult to resolve.4
Peri-implant mucositis is much like gingivitis around natural teeth in that they are both precursors to the more serious conditions of peri-implantitis and periodontitis, respectively—although neither necessarily will progress beyond a contained lesion. The major difference between teeth and implants in terms of susceptibility to disease progression is that teeth have supracrestal connective tissue fibers that insert into their root surface along with their surrounding circular fibers, whereas dental implants have only the latter. Without this additional protective mechanism, dental implants are less resistant to inflammation in the surrounding supracrestal connective tissue and rely strictly on the rather weak hemidesmosmal attachment of the sulcular epithelium to resist disease progression. Thus, care of these tooth replacements is all the more critical.3
Prevalence
It has been reported that approximately 10% of implants will develop peri-implantitis.5 It is difficult to establish a definite prevalence, however, because of significant variations in diagnostic parameters. Moreover, there is no consensus on how much bone loss must occur to classify early diagnosis and detection; there is also confusion as to whether the loss relates to other possible factors like physiologic modeling/remodeling after implant placement, implant position, occlusal overload, or a ridge-to-implant width discrepancy.
Etiology
The inflammatory processes of periodontal disease and peri-implant disease are traced primarily to bacterial plaque and calculus, which cause destruction to the surrounding bone and connective tissues, potentially leading to tooth loss and implant loss, respectively.3 In the case of peri-implantitis, several additional co-factors have been proposed in a recent publication, including occlusal forces, damage to the implant surface, cement, and corrosion that could work synergistically with bacteria to progressively increase the loss of supporting bone.6
Hygiene Considerations
Hygiene efforts aimed at the treatment or prevention of periodontal disease can be hindered by the existence of root anomalies that complicate scaling and root planing. As noted by Gher and Vernino, root morphology is an extremely important factor in the treatment of periodontal disease, as the shape of the roots, presence of a furcation, or the existence of anomalies like enamel projections or grooves may contribute to development of periodontal defects by providing an environment favorable to the retention of plaque.7 Furthermore, increased pocket depth can also be a limiting factor to the complete removal of plaque and calculus from the root surface.8 All of these issues may dictate a surgical approach to care to help ensure removal of plaque and calculus from root surfaces.9
Dental implants, like teeth, can also provide certain challenges to plaque removal. Depending upon available bone, dental implants may be placed in positions that require prosthesis fabrication that hinders hygiene efforts. For example, ridge resorption following extraction may require more apical or lingual/palatal implant placement, necessitating crown contours that make access difficult forhygiene. Moreover, in an effort to provide esthetics, pink porcelain or ceramic is often used, which can obviate effective oral hygiene. Another challenge occurs if there is bone loss on the implant leading to exposure of the roughened surface or perhaps even the threads, both of which can increase plaque retention.
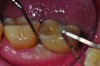
Treatments aimed at controlling inflammation and infection and limiting biologic complications for peri-implant mucositis and peri-implantitis may be identical to those found effective for gingivitis and periodontitis, respectively. Similar to gingivitis, peri-implant mucositis, when detected early, may be successfully treated with effective nonsurgical efforts aimed at elimination of the biofilm from the implant surface10-12 (Figure 5 through Figure 7), through supportive periodontal therapy and mechanical nonsurgical treatment.
However, if the roughened implant surface or threads become exposed, these situations may require implantoplasty—ie, smoothing the outer surface of the dental implant—with burs to facilitate hygiene efforts and reduce plaque accumulation. Perhaps the most important distinction between peri-implant mucositis and peri-implantitis in terms of their clinical implications is that peri-implant mucositis has been shown to respond to nonsurgical treatment,10 whereas peri-implantitis does not.13 Eliminating peri-implantitis may require that a specialist perform advanced technique sensitive surgical procedures that include systemic antibiotics, regeneration,14,15 or combined layered approaches.16
A question often asked is, what are the best instruments to clean around dental implants? There is no one ideal device or method that has been elucidated through a review of the literature.17 The most important point to be made is that implant sites need to be debrided at every recall visit using whatever device might remove the plaque or calculus present while limiting damage to the outer implant surface. In some instances, this may mean that a plastic instrument or one made from titanium may suffice. In other situations, titanium brushes, stainless steel instruments, or subgingival use of air powder abrasion may be necessary.
Prevention
In view of what is known about peri-implantitis—its commonalities and association with periodontitis, the various degrees of bone loss, and the risk it poses to implant or restoration survival—every effort should be made to prevent peri-implant mucositis. The first line of care is to identify possible risks to mucositis development. Among those reported in the Consensus Report of the Sixth European Workshop on Periodontology were prior periodontal disease; poor plaque control and inability to clean due to position of the implant; occlusal overload; genetic predisposition and conditions such as diabetes and cardiovascular disease; and smoking.1 Most recently, factors such as retention of cement and cardiovascular disease18 have gained much attention. When possible, eliminating those factors that can be controlled should be pursued. For example, pre-existing periodontal disease should be resolved prior to implant placement, as studies suggest that residual pockets harbor periodontal pathogens that can infect other sites.19,20 Where this is not possible, a maintenance plan must be implemented that accounts for the higher risk factors associated with these conditions. In the case of adverse prosthesis design, there should be more frequent recall care—ie, patients should be seen at intervals of 2 to 3 months—and prosthesis retrievability through screw retention must be considered. Periodic removal of screw-retained prostheses may be necessary to enable the hygienist to access plaque retentive areas.
Early Diagnosis
When preventive measures fail and problems arise, it is imperative to recognize and render treatment at the earliest stage possible. This requires prompt diagnosis, which can best be accomplished through meticulous record-keeping and close monitoring to gather the pertinent information regarding bone and tissue abnormalities that signal the onset of peri-implant mucositis or its progression to peri-implantitis.
Radiographs
The authors believe, in accordance with the American Academy of Periodontology’s whitepaper,21 that clinicians should obtain radiographs that establish baseline bone levels both at the time of implant placement and immediately following final prosthesis insertion to facilitate comparison. They should also be taken at the first signs of disease that could signal bone loss, including change in tissue tone and color, bleeding, purulence, and increased or increasing probing depths—all of which should be evaluated at every visit.21
Probing, Bleeding, Suppuration
Initial probing of the implant should be done once the final restoration has been placed and can be done with a periodontal probe that is either plastic or metal. The key to consistency is to use light force with the same type of probe, with probing depth and clinical attachment levels being recorded. Probing depth is defined as the depth of probe penetration from the base of the implant sulcus to the crest of the gingival tissue surrounding the implant, whereas attachment level involves using a fixed reference point—eg, crown margin—to make these measurements.
Recall Visits
The authors suggest that clinicians otherwise employ methods that monitor implant health and determine inflammatory complications as part of an ongoing periodontal maintenance program, which should include a comprehensive periodontal and implant examination. As noted above, because implants differ from natural teeth, they should be subject to more frequent recall visits than the twice-yearly interval recommended for teeth, perhaps alternating between the restorative dentist and periodontist who placed the implant. Moreover, in patients with known risk factors, it becomes imperative that their visits be increased to at least three to four times per year.
Patient and Professional Education
Hygienists are in a unique position to monitor changes in a patient’s health history or clinical situation (new prosthesis placement) that make their patients more susceptible to peri-implant disease development. In addition to efforts to eliminate periodontal disease prior to implant placement, they should identify patients with conditions such as diabetes and hypertension, if possible, and explain their susceptibility and the importance of receiving treatment to control these contributing factors. Hygienists should also attempt to review and reinforce good oral hygiene to assure optimal cleaning of the prosthesis. They should also try to encourage smoking cessation among those patients who smoke and monitor patients for brushing or parafunctional occlusal habits like clenching.
All members of the dental team should be aware that:
• pockets of 5 mm or greater are a significant risk factor for peri-implant disease and that a radiograph should be taken when patients present with bleeding or suppuration that is not due to prosthesis design.
• patients who receive treatment for the elimination of peri-implant disease should be reevaluated at a scheduled follow-up visit soon after to determine the success of elimination efforts.
• adjunctive care for mucositis may include air powder abrasion, local antibiotic delivery, and/or laser procedures.
• when nonsurgical methods do not successfully resolve peri-implant disease, surgery should be considered.
Most importantly, the dental team should receive continuing education on this subject as prevailing understanding and treatment may change as both dental implants and the field of implantology continue to evolve.
Referral
For general practitioners who are placing dental implants, part of long-term implant survival may well mean collaborating with a periodontist, especially if the physiologic modeling and loss of marginal bone after implant placement result in the implant’s roughened surface and/or threads becoming exposed. Technique-sensitive surgical procedures and alternating recall visits may be essential to assure that the implant can be restored to and maintained in a steady state of health. It is important that both the restoring dentist and the periodontist take the time and care to update records and ensure that these sites have been thoroughly debrided.
Conclusion
As with many inflammatory diseases, the best treatment outcomes are associated with early diagnosis and treatment. Toward that goal, clinicians should plan for success with implants by following guidelines designed to prevent or minimize the potential for the development of peri-implant disease and, if necessary, be prepared to effectively diagnose and treat it.3 This means screening patients for risk factors associated with developing peri-implant diseases; taking care to fully treat periodontal disease, which can infect implant sites; employing methods that support and monitor implant health, and recognize inflammatory complications as part of an ongoing periodontal maintenance program. It is far less debilitating and more cost/time effective to save an implant than to remove and attempt to replace it.
References
1.Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(Suppl. 8):282-285.
2.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance workgroup. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-920.
3.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000 1998;17:63-76.
4.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11-25.
5. Mombelli A, Müller N, Cionca N.The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23 Suppl 6:67-76.
6. Mouhyi J, Ehrenfest DMD, Albrektsson T. The Peri-implantitis: Implant Surfaces, Microstructure, and Physiochemical Aspects. Clin Implant Dent and Related Research. 2012;14:170-183.
7. Gher ME, Vernino AR. Root morphology, clincial significance in pathogenesis and treatment of Periodontal disease. J Am Dent Assoc. 1980;101:627.
8. Stambaugh RV, Dragoo M, Smith DM, Carasali L. The limits of subgingival scaling. Int J Periodontics Restorative Dent. 1981;1:30-41.
9. Brayer WK, Mellonig JT, Dunlap RM, et al. Scaling and root planing effectiveness: the effect of root surface access and operator experience. J Periodontol. 1989;60(1):67-72.
10.Heitz-Mayfield L, Salvi GE, Botticelli D, Mombelli A, Faddy M, Lang NP. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22:237-241.
11. Pontoriero R, Tonetti MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced periimplant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254-259.
12. Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182-190.
13. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36:1600-1605.
14. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
15. Froum SJ, Rosen PS. Re-entry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 2014;34(1):47-59.
16. Schwarz F, Hegewald A, John G,et al. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol. 2013;40:962-967.
17. Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23(6):643–658.
18. Renvert S, Aghazadeh A, Hallström H, Rutger Person G. Factors related to peri-implantitis – a retrospective study. Clin Oral Implants Res. 2014;25(4):522-529.
19. Levy RM, Giannobile WV, Feres M, et al. The effect of apically repositioned flap surgery on clinical parameters and the composition of the subgingival microbiota: 12-month data. Int J Periodontics Restorative Dent. 2002;22(3):209-219.
20. Matuliene G, Pjetursson BE, Salvi GE, et al. Influence of residual pockets on progression of periodontitis and tooth loss: results after 11 years of maintenance. J Clin Periodontol. 2008;35(8):685-695.
21. American Academy of Periodontology Task Force on Peri-implantitis. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84(4):436-443.
About the Author
Paul S. Rosen, DMD, MS
Clinical Associate Professor of Periodontics
University of Maryland Dental School
Baltimore, Maryland
Private Practice
Yardley, Pennsylvania
Stuart J. Froum, DDS
Clinical Professor and Director of Clinical Research
Department of Periodontology and Implant Dentistry
New York University College of Dentistry
New York, New York
Private Practice
New York, New York