You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
According to the US Surgeon General’s report on Oral Health published in 2000, the mouth—including the teeth, gums, and supporting bone structures—is a “mirror for general health and well-being.” In the report, periodontal disease is also described as the second most prevalent disease in the United States (the first being heart disease), and the leading cause of adult tooth loss.1
In addition, a 2002 poll of 1,000 people over the age of 35 conducted by Harris Interactive Inc (Rochester, NY) found that 60% of adults surveyed knew little, if anything, about gum disease, the symptoms, available treatments, and—most importantly—the consequences. Additionally, 39% do not visit a dentist regularly.2
Across all demographic categories in the United States, 50 million people have some form of gum disease, and another 85% to 90% of the population has gingivitis, which may be a precursor to periodontal disease.3,4
Of course, not all cases of gingivitis will progress to periodontal disease, but there is a definite imbalance between the small amount of periodontal therapy that is being provided with regard to the great amount of periodontal disease that exists in the population. Gingivitis usually precedes periodontitis, but not all gingivitis progresses to periodontal disease. Gingivitis is initially caused by plaque build-up, the clinical manifestation of which is inflamed gingiva that bleeds easily during flossing and sometimes during brushing. The teeth are still firmly seated—there is no attachment loss associated with gingivitis. Periodontitis progresses in stages, and its clinical manifestations include varying degrees of receding gums, gingival attachment loss, measurable pocket formation, and infection.
There are basically two models of periodontal disease. The classic model is a linear progression that starts at health and moves to gingivitis, then periodontitis, with its associated bone loss, bleeding, and radiographic evidence of disease. Today, the profession has come to understand a new model that, like the old one, still moves in a linear fashion, but also branches off to the side and even does a little backtracking; the new model is more dimensional and multifaceted than before.5
It is now known that the bacterial species consistently associated with periodontal disease are Porphyromonas gingivalis, Treponema denticola, Tannerella forsythensis, and Actinobacillus actinomycetemcomitans, and it is not the bacteria themselves that cause damage to the human body, but rather the enzymes that the body produces in response to these bacteria that are to blame.6
The body’s immune system responds to the overwhelming influx of pathogenic bacteria in the mouth, on teeth and gums, and in gingival crevices by initiating an inflammatory response. The inflammatory response is precipitated by a release of certain enzymes that rush to the infected areas to combat the bacteria and their bacterial endotoxins. These enzymes include collagenase, elastase, gelatinase, and proteases, which are destructive to the connective tissues of the gingiva. For example, elastase and collagenase break down and destroy elastin and collagen, which are the primary structural components of the periodontium. Gelatinase destroys the gelatinous matrix that surrounds the biofilm, and proteases destroy proteins. This represents much destruction, and it is concentrated within the connective and supportive tissues of the gingiva. This is not to say that bacterial burden does not contribute to the destruction of tissues through the release of a variety of toxins and endotoxins, but these are not nearly as destructive as the damage caused by the body’s own inflammatory response.
There is a wealth of information available that clearly demonstrates an association between the health of the mouth and the health of the body.7 However, more research needs to be done, particularly in the realm of interventional studies, which may very well indicate some causal link between periodontal disease and systemic diseases and conditions.
Even though oral healthcare providers and medical professionals know the potential health consequences of periodontal disease, there are certain things that can be done to elevate the standard of care when it comes to treating periodontal disease. As oral healthcare providers, it may be easier to elevate a personal standard of care by adopting a simple five-step plan that includes diagnosis, treatment, appropriate fees, and referrals. This simple process will enable professionals to navigate within this vast and often complex territory called periodontal disease and ensure better patient outcomes.
Step 1: Diagnose Confidently
The prevalence of periodontal disease is quite significant. When gingivitis is included, close to 100% of the population is affected. So the question remains: why are oral healthcare providers not treating everyone for periodontal disease or providing preventive care in the early, non-surgical treatment stages? One reason for this is that, once periodontal disease is diagnosed, the patient may ask, “I come in every 6 months. How come all of a sudden I have this problem?”
That is a legitimate question that requires a compelling answer. Here is one approach: “That is a very good question, Mrs. Smith, and I understand your surprise and concern. Up until recently, we thought that periodontal disease was a gradual disease. However, today’s emerging research indicates that periodontal disease really occurs in spurts, or bursts of activity, that can wax and wane. Therefore, there are periods of healing, followed by periods where the disease wins out over the body’s own defense mechanisms. But the end result is that, over time, the disease continues to get worse.”
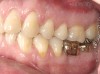
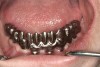
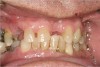
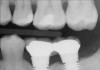
It is also important to explain to patients that the health of their gums is assessed every time they come in; but at this point in time, there are some areas of bleeding on probing, with pockets of disease around their teeth greater than 3 mm deep. It must also be communicated that this is a sign of infection in their gums and that it should be treated immediately (Figure 1).
Another scenario involves examining new patients, and discovering they have periodontal disease. New patients commonly react by becoming defensive, saying, “How come my former dentist didn’t diagnose this?” In cases such as this, it is important to respond tactfully: “That is a very good question, Mrs. Smith. Did Dr. Jones do all of the same assessments that we did here today, with measurements, and bleeding points, and pocket depths?” Inevitably, the patient responds “no,” but in the unlikely event they do say “yes,” that is fine, too. The author’s recommended response would be: “That would be a very good historical record for us to have in your files, so we can see the progression that has occurred over time, because everyone’s progression is different. And that’s an important thing for us to know as we go forward to take care of your gum disease.”
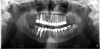
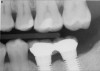
There are several ways to diagnose periodontitis. In addition to soft tissue evaluation, one of the most common and accurate methods is to use a periodontal probe in affected areas and record probing depths. Bleeding on probing is another indication of unhealthy periondontal tissues. Radiographs can be used, but they have limitations in initial diagnosis because bone loss is typically associated with severe and chronic periodontal disease; therefore, radiographs are better used to confirm diagnosis. However, vertical bitewings taken over time can be placed side-by-side to detect even slight differences in contrast.
Gingival recession can indicate attachment loss, which can vary from tooth to tooth. However, a tooth can have attachment loss without having a pocket. Tooth mobility tests may also be performed. In this case, the non-working ends (handles) of two dental instruments are pressed against the buccal and lingual surfaces to detect movement, which is categorized in classes: Class O is a completely stable tooth; Class I is 0.5 mm of buccal and lingual movement; Class II is all degrees of movement > 0.5 mm and < 1 mm in any direction; and Class III indicates movement > 1 mm in any direction and is depressable in the socket. In advanced (severe) periodontal diasease, furcation probing is important and a reliable diagnostic technique. A Naber’s probe is used, typically on multi-rooted teeth, to determine furcation involvement.
Step 2: Treat Promptly
Once the proper diagnosis has been established, the next step is treatment planning. In the author’s opinion, treatment delays stem primarily from not having a systematized, organized periodontal treatment protocol in place. Treatment protocols must be easily implemented and easy to put into practice. Ideally, the protocol should be one that can be choreographed in an appointment-by-appointment fashion, organized by disease state and/or pocket depth. Sample scripting that gives examples on how to talk with patients each step of the way can be helpful, as are listings of the proper insurance codes to maximize patient benefits for each phase of treatment. This level of orderliness is also the key to maximizing profitability.
Treatment protocols vary and should be assessed on a case-by-case basis. There are five periodontal disease classifications: Type 1, Gingivitis; Type II, Mild Periodontitis; Type III, Moderate Periodontitis; Type IV, Advanced Periodontitis; and Type 5, Refractory Periodontitis. Patients in the Type II through V range will initially require scaling and root planing, in addition to oral hygiene instructions and patient education. In pockets > 5 mm, locally applied antibiotics may be beneficial to help eradicate the bacterial source of infection, as well as promote tissue healing. Six to eight weeks after periodontal scaling and root planing (to allow sufficient time for healing), the patient should return for a reevaluation. Clinical findings, including probing depths, from the patient’s initial examination are compared to the reevaluation findings. Subsequent treatment can proceed in several ways, depending on the initial response. This may include additional nonsurgical therapy sessions, supportive periodontal care and maintenance, or surgical treatment. Again, the treatment plan will vary and should be based on ongoing clinical assessment and individual patient response to treatment.
Another reason oral healthcare professionals avoid or delay treating periodontal disease is the fact that the success of the treatment depends so much on the success of patients’ compliance with bacterial control at home: plaque control, brushing, flossing, etc. Collective experience indicates that patients do not comply, and non-compliance is a critical underlying issue. Because compliance is clearly a major issue, how do oral healthcare providers encourage it? Encouraging patients to comply with a home-care regimen is delving into behavior modification territory.
Volumes have been written about compliance and behavior modification in the medical industry, with notable research into compliance in regard to taking prescribed medication.8 Research has shown that compliance increases as the number of pills required per day decreases. For example, in a study of people taking medication for cardiovascular conditions, the average adherence was 95% for once-per-day dosing, 84% for two- and three-times per day dosing, and 78% for those taking four pills per day.9 Subjects who were on a once-a-day regimen had an average compliance rate of 83% compared to 53% for those on a twice-daily regimen and 27% for those on a four-times-per-day regimen.9
The fact is, the less a patient is asked to do, the greater the compliance. This can also be applied to the amount of oral home care prescribed. If it is recommended that a patient do too many things (ie, brushing, flossing, irrigation, using tongue scrapers, rubber tips, electric toothbrush, etc), they will inevitably do only one or two of those things and let the others fall by the wayside. That is why it may be best to ask them to do the two most basic and important things—brush and floss—and show them how to do it well enough to make a positive difference in their oral health.
Another method is to create an emotional impact to serve as an impetus to change patient behavior and compliance for the better. One effective way to charge patients to do their part is to scrape the dorsum of their tongue with a plastic spoon, or even a mirror, and allow them smell it. One way to speak to a patient about this subject can begin like this: “Mrs. Smith, this is a primary bacterial population that is growing on your tongue, and it is the same bacteria that is growing in your gums.”
Next, take a thick piece of floss and take several passes in between the first and second upper molars. Then remove the floss and let them smell it. There will likely be more compliant flossers when patients smell how much halitosis is coming from those areas.
Counting and tracking bleeding points is another strong compliance motivator. Let patients know a record of their bleeding sites is being kept: “You have 12 in the upper right, 16 in the lower right, 11 in the upper left, and 9 in the lower left, for a total of 48 bleeding sites.” At the next appointment, count the sites again, and when they have fewer sites on their chart, give them positive reinforcement: “You are down to x-number of bleeding sites; that is a great improvement, Mrs. Smith. You are getting that infection under control. Good for you.” This is behavior modification in action.
Step 3: Treat Comprehensively
For periodontal disease treatment to be as successful as possible, patient compliance with respect to diligent home care is a major factor. After scaling and root planing in the office, the patient is sent home and the healthcare provider hopes for the best. In addition to a prescribed home care regimen, antibiotics may be prescribed.
Systemic antibiotics have been used for decades and they do work, but usually they are prescribed in short-term “blasts.” Combinations of drugs, such as a 2-week course of doxycyline (100 mg, once a day) along with surgical or non-surgical debridement can lead to stabilization of the disease process. However, it appears that tetracycline-resistant strains of pathogenic bacteria are beginning to emerge10,11 and, hence, some patients will respond better to a combined course of amoxycillin (250 mg three times a day) and metronidazole (200 mg three times a day) for 7 days.12
The author advises against prescribing too many systemic antibiotics when there are other infection treatment modalities, including locally administered antibiotics such as minocycline hydrochloride 1 mg which is proven safe and effective, without building up antibiotic resistance.5,13 Other site-specific antibiotics for the treatment of periodontal disease include doxycycline hyclate, 10 mg, and chlorhexidine gluconate, 2.5 mg.
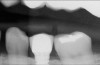
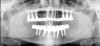
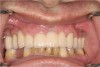
The goal is to avoid systemic drugs if possible, so as to not build bacterial resistance. As mentioned before, compliance can be a problem with prescription medications. When locally administered antibiotics such as minocycline hydrochloride are used, there are no compliance concerns. The patient is treated chairside, and the antibiotic is syringed into each pocket, where it stays and continues to provide antibiotic action for up to 30 days.14 Figure 2 depicts the administration of a locally applied antibiotic. Locally administered antibiotics have an advantage in that they are targeted right at the source of infection: the antibiotic is absorbed right through the epithelium on contact, so there are effective therapeutic levels exactly where they are most needed.15,16 Systemic antibiotics pass through the digestive system, where much of the active medication is absorbed and metabolized before it even reaches the target area. Unlike systemic antibiotics, where blood concentrations of the drug continue to lessen as they are metabolized in a short amount of time, locally administered antibiotics such as minocycline hydrochloride can provide a sustained release of a constant, effective level or concentration of the drug for 2 weeks or longer.14
Step 4: Charge Accordingly
“How much is my insurance going to pay for?” This is the question dental professionals hear most often from patients, and the author thinks that is the foundation of the widespread fear of charging for periodontal treatment. Patients need to be educated about dental insurance. Dental insurance is “payment assistance;” it is meant to help offset the cost of treatment, not pay for it entirely.
In the author’s opinion, in the early 1980s, the profession became accustomed to using a great “closer” to get patients to accept dental treatment: “Don’t worry, your insurance will pay for that.” That statement worked back then because, for the most part, insurance coverage was closest to actual fees for service. With the advent of PPO and DMO plans, the fees paid for dental services to participating dentists are effectively reduced by contract as a cost containment measure.17 Today, insurance companies continue to try to reduce their own costs by attempting to alter treatment plans and thus limit treatment by raising the patients’ financial contribution.18 However, there has been some progress made with insurance companies, as reported by The New York Times in November 2006. For example, some insurance companies, particularly Aetna Dental and CIGNA, are now paying for additional and adjunct periodontal maintenance therapies because it may reduce the cost of more significant treatments for heart disease, pre-term birth, and other systemically linked illnesses.
Another insurance hurdle is the fact that most dentistry is done “one tooth at a time,” and most dental care can be characterized as the “crown-of-the-year club,” meaning that dental insurance will cover about one crown a year, so dentists will find the worst tooth and crown it this year.
However, treating and charging for periodontal disease is very different. In the case of a crown, once the crown is cemented, the treatment has reached an end point. In contrast, periodontal disease is an ongoing, never-ending disease that dentists can control but cannot cure. This is another aspect of periodontal disease that the profession must change—it has been downplaying the disease as a whole and glossing over it; instead of explaining it to the patient, it is simply called a “cleaning.” A cleaning is covered twice a year, but it is obviously not comprehensive periodontal treatment.
There are many specific things performed during a patient’s hygiene experience, and it should never be downplayed as simply “a cleaning.” Dental professionals do not “clean” teeth; they perform preventive prophylaxis or therapeutic treatments. The profession needs to change the way this is discussed, and validate the fee in treatment planning. Because, in the minds of the patients, if it is just a cleaning or a check-up, they may procrastinate on accepting and following through with the recommended treatment plan.
Step 5: Refer Wisely
For many general practitioners, referring a patient to a periodontist is a limited option because many dental practices have not taken the time to build a solid working relationship with periodontists. Communication is essential so that everyone is always on the same page.
The author has spoken with periodontists who say that their general practitioner has sent patients to them for periodontal surgery, however the patients were not ready for surgery because there was inflammation and disease that needed to be addressed beforehand. These patients would most likely be confused and upset, because they had been led to believe that they were ready for surgery. In this case, the periodontist could explain: “Dr. Smith did what he could within the limits of his practice, but now we are going to do it in a specialty practice at a level that is pre-surgical.”
Both the periodontist and general practitioner should always know exactly what the other is going to say on the matter. A general practitioner who simply tells the patient, “We’re going to send you over to the surgeon now” is asking for trouble. A better way to communicate is: “You deserve some further attention and I work with a specialist, so let’s get his/her input before we go any further.”
There are two treatment plans for a patient with advanced periodontal disease—one is set by the general practitioner, and the other is set by the periodontist—and for both of them to work as they should, communication must be open and often. They should look at radiographs together, plan treatment together, and set both short- and long-term goals for the patient.
Lastly, consider opening up lines of communication with physicians, especially if a patient’s overall systemic health may be in some way impacted by oral health status, such as patients with diabetes, heart disease, or other prevailing systemic conditions. Doing so is a good way of educating physicians about the systemic links and other emerging research in the dental field. When patients start to hear about the oral/systemic connection not only from their dentist, but their doctor, too, it adds emphasis and another dimension of credibility: “My dentist always told me I needed to take better care of my teeth and gums, and now my doctor is telling me the same thing—I better start taking this more seriously.”
Conclusion
Periodontal disease is known as a “silent” disease because patients do not know they have it (or simply ignore the symptoms) until it has reached an advanced state. Patients will often go months and months with bleeding gums and not seek treatment, while bleeding from any other body orifice would immediately send them to their physician.
Not seeking care is likely a result of a number of factors. The first is that pain or discomfort does not occur until the latter stages of the disease, so the patient concludes that a “little bleeding” around the gums cannot be too serious. Personal reasons, including fear and embarrassment, may also come into play. Or they simply just do not realize the scope of the disease or its potential impact on whole-body health.
If the patient had a nose bleed every day, he or she would surely seek medical attention. With periodontal disease, the imperative need for treatment is not as obvious. Once patients realize that they have active infection in their mouths, it is usually a wake-up call they can understand.
Because patients do not or will not take notice on their own accord, it is up to dental professionals to become the front line of awareness for their patients. All dental practices should establish a periodontal treatment protocol that works best for them, and the entire staff should be involved and “on the same page,” yet clear about their specific roles. Now is the time to take a closer look at the specific “fears” surrounding the failure to diagnose, treat, charge, and refer patients with periodontal disease; recognize the areas in need of improvement; and focus on implementing this five-step plan for elevating and committing to your personal standard of care.
Disclosure
The author has no financial interest in any of the manufacturers mentioned in this article.
References
1. US Department of Health and Human Services, National Institutes of Health, National Institute of Dental and Craniofacial Research. Oral Health in America: A Report of the Surgeon General. Washington, DC: May 2000.
2. Lewis C. Fighting gum disease: how to keep your teeth. FDA Consum. 2002;36(3):16-22.
3. Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994 [published corrections appears in: J Periodontol. 1999;70(3): 351]. J Periodontol. 1999;70(1):13-29.
4. Coventry J, Griffiths G, Scully C, Tonetti M. ABC of oral health: periodontal disease. BMJ. 2000;321(7252):36-39.
5. McLeod DE. A practical approach to the diagnosis and treatment of periodontal disease. J Am Dent Assoc. 2000;131:483-491.
6. Dean JW, Branch-Mays GL, Hart TC, et al. Topically applied minocycline microspheres: why it works. Compend Contin Educ Dent. 2003;24(4):247-257.
7. American Academy of Periodontology. Parameter on systemic conditions affected by periodontal diseases. J Periodontol. 2000;71(5 Suppl):880-883.
8. Mitchell JH. Compliance with medical regimens: An annotated bibliography. Health Educ Behav. 1974;2:75-87.
9. Dunbar-Jacob J, Bohachick P, Mortimer MK, et al. Medication adherence in persons with cardiovascular disease. J Cardiovasc Nurs. 2003;18(3):209-218.
10. Fiehn NE. Doxycycline-resistant bacteria in periodontally diseased individuals after systemci doxycycline therapy and in healthy individuals. Oral Microbol and Immunol. 1990;5(4):219-222.
11. Feres M, Haffajee AD, Allard K, et al. Antibiotic resistance of subgingival species during and after antibiotic therapy. J Clin Periodontol. 2002;29(8): 724-735.
12. Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37(5):389-398.
13. Preshaw PM, Hefti AF, Jepsen S, et al. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J Clin Periodontol. 2004;31(9):697-707.
14. Goodson JM, Gunsolley JC, Grossi SG, et al. Minocycline HCL microspheres reduce red-complex bacteria in disease therapy. J Periodontol. 2007;78(8):1568-1579.
15. Kinane DF. Local antimicrobial therapies in periodontal disease. Ann R Australa Coll Dent Surg. 2000;15:57-60.
16. Hanes PJ, Purvis JP. Local anti-infective therapy: Pharmacological agents. A systematic review. Ann Periodontol. 2003;8(1):79-98.
17. Gluck G, Aroskar M, Arthur N. Cost containment in dentistry and its impact on the distribution of services. Theoretical Medicine and Bioethics 1983;4(2).
18. Cooney PV, Hassard TH, Ulla A, Spangen DA. Predetermination as a Cost-containment Mechanism in a Social Allowances Dental Program in Manitoba. Journal of Public Health Dent. 195;55(3):177-180.