You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Lesson
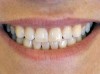
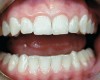
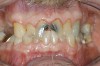
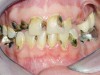
Caries risk assessment is a hot topic in the dental profession today. With the introduction of caries risk-assessment principles, dentists are now asking, "Is it possible to prevent cavities before they start?" This is a complex question and it is one that is getting some serious attention as the dental profession begins to understand biofilm disease models and their role in the dental caries process. Caries risk assessment, or CAMBRA, an acronym for "caries management by risk assessment," is striving to become the standard of care in dental schools and a growing number of dental practices. Simply put, caries risk assessment is a philosophical approach to dental caries that involves examining the specific risk factors responsible for the disease process in each individual and applying measured treatment trategies directed to correct or modify those risk factors. In this sense, caries risk assessment encompasses diagnosing and treating the cause of the symptoms, not just the endless surgical repair of the signs and symptoms of the disease. Patients are diagnosed as low, moderate, high, or extreme risk for caries; their risk factors are identified and their caries activity level is assessed (Figure 1 through Figure 4).
HISTORICAL PERSPECTIVE
The dental profession originally approached dental caries with very traditional philosophies and strategies based on the known disease models at the time. At the turn of the 20th century there was a rapid age of discovery in dental caries as James Leon Williams first demonstrated the demineralization of enamel under a gelatinous plaque mass.1 Within 3 years, GV Black concluded the demineralization was caused by the lactic acid dissolving the calcium and phosphate salts from the enamel structure.2 These discoveries led to an attempt to make dental caries a pathogen-specific disease that fit our understanding of the disease process at the time. Based on these discoveries, the etiology of dental caries was based on acidogenic/aciduric bacteria. Mutans streptococci and Lactobacilli were identified as the causative agents and became the focus of future scientific studies. In the early disease model, the processes were limited to identifying a single or, in this case, two pathogens. Based on his understanding of the disease process at the time, Black created systems and standards for restorative dentistry while advising the profession on the importance of understanding the pathology of dental caries. Effectively, treating dental caries became a surgical intervention with the removal of diseased enamel and dentin followed by an attempt to restore the defect with a multitude of materials. The treatment was limited by the simplicity of the profession's disease model of the time. Unfortunately, it is now known that the surgical treatment for dental caries has little if anything to do with treating the disease, but has only short-term benefits against the pathogens, while restoring function to the teeth and relieving pain.3
SCIENTIFIC BASIS
Because the dental profession first identified and implicated mutans streptococci and Lactobacillus as the causative agents in dental caries, there has been a steady evolution of scientific studies adding to the knowledge base. In the early 1980s research began to demonstrate that levels of mutans streptococci and Lactobacilli were directly linked to caries incidence. It is important to note that there is great confusion and inconsistency in the dental profession in the use of the terms "dental caries" or just "caries."While it is most correct to use the terms to describe the disease or disease process, which includes all of the factors responsible for the expression of the signs and symptoms, frequently dentists and researchers use the terms to identify or describe the cavitations or other signs and symptoms. Better put, a hole or cavity in a tooth is a cavitation, which is a sign/symptom, while dental caries is a biofilm disease. That said, early work by Zickert et al concluded that not only was the incidence of dental caries related to levels of mutans streptococci and Lactobacilli by themselves and in combination, but children with high levels of these bacteria developed significantly more new cavitations than children with low levels of these bacteria.4,5 During the scope of their studies, children with high bacterial levels developed 20.8 new cavitations vs 3.4 cavitations in children with low bacterial levels.6 This continued to support the earlier bodies of work identifying these two pathogens in the caries process.
Further studies began examining the effect of controlling the bacterial levels and a resulting reduction in caries (cavitation) incidence. Dental caries is also known to be both infectious and transmissible, and studies have demonstrated transmission from mother to child7 and, in fact, to and from every member of a family unit. Today, primary caregivers of infants may be a relative other than the mother or a paid nanny or au pair. They too can transmit the disease to children. Additional studies indicate that the age of onset and severity of exposure to these bacteria are the greatest predictors in the childhood caries experience. Reduction in the mutans streptococci and Lactobacilli levels in the mother reduces the caries incidence in a child.8 This has appropriately led to prenatal counseling strategies for expectant mothers, because often the best protection for the newborn is reduction of the disease in the mother.
More recently, Featherstone introduced the concept of the caries balance,9 and demonstrated that managing dental caries by caries risk assessment does work. In a landmark study, he demonstrated a significant reduction in new cavitations for patients who were treated with caries risk assessment and a medical model vs patients who were treated with the traditional surgical model of restorative dentistry.10 Medical management of dental caries is possible and it provides better treatment outcomes than surgical intervention alone.
THE BIOFILM DISEASE MODEL
Looking beyond the traditional disease model with a single pathogen, it is now understood that dental caries is a complex, multifactorial disease with the oral microbial component being a biofilm rather than being planktonic in nature.11 When factors in the oral environment favor these bacteria, the biofilm population shifts from the normal, healthy flora to the acidogenic, aciduric bacteria associated with dental caries.12-14 A biofilm is present in nature whenever there is fluid, a surface, and bacteria present. Certain species of bacteria, early colonizers, attach to the surface, and convert from planktonic to sessile. In this process, they undergo up to 84 gene changes, which significantly changes their behavior. The biofilm is a sophisticated ecosystem composed of a multitude of bacterial species contained within its own infrastructure. There are metabolic and waste channels, the component bacteria share genetic information and communicate with each other. Within the biofilm, the bacteria may now become up to 1,000 times more resistant to antibodies, antibiotics, and antimicrobial materials.
The biofilm disease model represents a significant new challenge in managing and treating dental caries. Previous scientific studies that examined the cariogenic bacteria as planktonic organisms now may not translate well to a biofilm disease model. The clinical studies remain relevant, however, as the researchers were involved in treating a biofilm disease in vivo. Examining biofilm research from environmental studies, including river streams to petroleum pipelines, several effective strategies have been established in treating biofilm difficulties. Mechanical debridement, heat, and a strong oxidizing agent are all effective strategies to disrupt an established biofilm.11 There are some obvious limitations in applying these same strategies to the oral cavity. In the mouth, it is nearly impossible to completely debride the cariogenic biofilm away. The biofilm reforms within hours after it is removed and bacteria are ubiquitous in the mouth. The early colonizers, mutans streptococci, attach to the pellicle, and are not adequately removed by brushing and flossing.13 Heat can be applied to living issue, but temperatures required to denature bacteria also denature human tissue. Strong oxidizers are used in different areas of dentistry, but must be applied to the mouth with limitation and under strict conditions to be used safely.
The pathogenesis of the biofilm itself becomes a pH issue. Mutans streptococci and Lactobacilli are acidogenic and aciduric.12,13 Typically, these bacteria represent less than 1% of the oral biofilm, but under certain conditions a healthy biofilm can be replaced with a diseased one, and these cariogens then account for >96% of the biofilm. The selection pressure for this population shift appears to be pH-related. There are many risk factors that drive this population shift, including diet, saliva,medications, and home care. Metabolism of dietary refined carbohydrates by cariogenic bacteria results in lactic and acetic acid production. This lowers the pH of the biofilm. Compounded with other risk factors, the acidic pH becomes the selection pressure that results in dental caries, and signs and symptoms of the disease.15-17 They thrive in this acidic environment because they have the unique ability to pump the acid hydrogen ions back out of their cell to maintain intracellular neutrality in an acidic environment, and they have intracellular enzymes that can operate in low pH environments.14 This hydrogen-ion pump mechanism is shared with all acidogenic/aciduric bacteria, and many more bacteria may play a role in the dental caries process which the research community has yet to successfully identify. So these cariogenic, acidogenic, and aciduric bacteria share these unique traits, and become the dominant species in the diseased oral biofilm, leading to demineralization of the teeth.
MEASURED TREATMENT STRATEGIES
While the science is straightforward concerning the cariogenic biofilm, treating and replacing it with a healthy biofilm becomes a challenge that requires an accurate diagnosis of the risk factors that led to the diseased biofilm, and a regimen that may help to reverse the situation. As a multifactorial disease, caries risk assessment then plays a significant role in helping the clinician routinely identify the known risk factors for dental caries.18,19 Use of a standardized caries risk-assessment form for all patients adds a scientific measure to the diagnostic process. Such a form was published in the CDA Journal in March 2003,20 and is available from CDAfoundation.org, WCMID.com, and Carifree.com (Figure 5).
Because dental caries is a multifactorial, transmissible bacterial infection, it makes sense to approach treatment with multifactorial, antimicrobial strategies. Strategies that focus on treating or removing a single pathogen will have only limited effectiveness in treating biofilm diseases.15 Based on the accumulating body of scientific research, several diagnostic tools made their way to the market to culture mutans streptococci and Lactobacilli from the saliva or tooth surface, examine pH changes of the plaque,21 or measure the adenosine 5'-triphosphate (ATP) of the biofilm (Figure 6). The bacterial cultures typically sample bacteria from the saliva and extrapolate the colony-forming units on the bacterial plaque.8 While it makes good sense to have a metric to measure for and quantify the presence or risk based on the pathogenic bacterial load, how best to accomplish that is still in question. Strict dependency on use of bacterial cultures has been called into question, although they have the benefit of patient education and motivation.22 The pH and ATP tests look promising and are currently being investigated but they both lack published studies. In the near future, clinicians should have a simple chairside metric to measure the bacterial biofilm component to aid in the diagnosis process.
On the treatment side of the equation, good home care with plaque control is important, but, additionally, clinicians have been using fluoride rinses and chlorhexidine rinses as antimicrobial strategies to treat the bacterial infection. Even povidone iodine has been used as an antimicrobial mouth rinse to fight this disease.23 Reversing the pH of the biofilm has been demonstrated to reduce the levels of pathogens24 and some clinicians have been using oral products based on reversing the pH selection pressure to correct the biofilm disease. Certainly xylitol products have been effective for some patients, and fluoride coupled with xylitol has demonstrated synergistic effects.25 Remineralization strategies also include products with amorphous calcium phosphate and casein calcium phosphate.26 Fluoride varnishes have been very useful materials to use for children,25 and 1,200/5,000 ppm fluoride dentifrices and gel also have an important role in caries treatment.27 Clinicians use a combination of treatment products that make sense for each individual patient based on their individual risk factors. The challenge is to keep the treatment strategy simple so that patients can be successful with compliance. A regimen that is too complicated, that uses too many materials on a complex schedule, is difficult for any patient to follow. It is best to use just a couple of products that can be implemented into the patient's normal hygiene routine, such as replacing regular toothpaste with a high fluoride content gel, and using an antimicrobial/fluoride oral rinse instead of mouthwash. Another good replacement therapy would be using high xylitol content gum for those patients who already chew gum. For patients that are frequent snackers, or have high refined carbohydrate intake in their diet, these issues need to be addressed and behaviors changed. Saliva plays a major role in oral health, and reduced saliva is a high risk factor. For many patients, the inadequate saliva flow may be a medication side effect, and for these patients, short of being able to change their medication, it is important to add therapy (eg, gum, rinses, and sprays) to compensate for the lack of saliva. The goal is to continue to add protective factors addressing the individual risk factors for the patient until the biofilm is healthy. Unfortunately, there is no magic pill for dental caries at present.
New scientific investigations involve short peptides coupled with antimicrobial agents as a strategy to eliminate a specific pathogen. These specific targeted antimicrobial peptides have been demonstrated in vitro to remove mutans streptococci from a biofilm.28 Whether mutans streptococci actually possesses the critical role the profession has attributed it to remains in question.29 And the value of removing one pathogen from a multipathogenic biofilm may have only limited value.17 Regardless of the outcome, serious steps are being made to develop treatment strategies to reduce the biofilm component from the dental caries disease process. The challenge for practitioners today is that there remains no known, documented, and established treatment formula for dental caries. Scientific studies under strict conditions have demonstrated improved treatment outcomes, but trying to duplicate and maintain these conditions in clinical practice has been difficult. The simple one-size-fits-all therapy that has worked well with a single-pathogen disease model may have only limited effectiveness with a multifactorial, multi-pathogenic, biofilm-based disease model. Short of a new phenomenal scientific breakthrough, the dental profession will need to continue focusing treatment strategies targeted to specific risk factors uniquely designed for each individual patient to be ultimately successful.
IMPLEMENTATION STRATEGIES
Like any new philosophy or technology, it is not easy to implement caries risk assessment into a dental practice, and it cannot be accomplished overnight. Caries management by risk assessment represents a huge paradigm shift regarding how dentists define, diagnose, measure, and treat the disease process.30 Implementing caries risk assessment will affect all of the systems in a dental practice including patient education, scheduling, and fees. The goal of caries risk assessment in private practice is for every patient to be educated on the cause of caries, or the signs and symptoms they know as cavities, the bacterial infection of the biofilm, and their risk assessment of factors that contribute to the disease and protective factors that balance risk. Finally, they need to understand the importance of treating the bacterial biofilm component simultaneous to restoring their cavities. Patients should be assessed on semi-annual or annual recare appointments for potential changes in their risk factors. It would make sense to screen for cariogenic bacterial levels on an annual basis as well. With these goals in mind, the next step is to determine the logical sequence of steps necessary to accomplish them.
First the dentist or leader must establish the vision and goals, and then the entire dental team needs to be educated on the importance of caries risk assessment as a standard practice. Like implementing any change in private practice, caries risk assessment goes nowhere without the support of the entire dental team. Once the team understands and supports the goal, each member can contribute to the roadmap design by identifying how caries risk assessment will change his or her responsibilities. This will create some new challenges, as the team evaluates how they can add more services into a fixed amount of scheduled time. Setting up a caries risk-assessment system in the practice requires some time and planning, and it is helpful for teams to spend some quality time preparing for the changes. There is no substitute for a firsthand experience, so it makes good sense for each team member to experience caries risk assessment as a patient. This may be an enlightening experience for the entire team, as each member may personally discover unknown risk factors or high oral bacterial loads. The profession has always used the "no cavities" as a gold standard for the measurement of health. But a patient with high risk factors and "no cavities" is just a patient with a disease that has not expressed symptoms yet.21 Conversely, a patient that does not have signs or symptoms is not necessarily at low risk. In the presence of a delicate balance, it may take only minor changes to create serious disease in what otherwise appeared to be a healthy mouth.
When the entire dental team is ready to implement caries risk assessment in the practice, the next step is to educate the patients. A personal letter explaining the new philosophy and benefits is a great way to break the news to everyone at the same time. Some practices have developed brochures explaining caries risk assessment and these are mailed with a cover letter to the entire patient base. Newsletters are another great way to get information out, with feature articles explaining caries risk assessment. Explain the sudden change in the practice's philosophy, and let them know what they can expect to be different on their next visit. Experience has shown that this is a very effective way to get detailed information across because most patients read their dentist's newsletters. The more information given to patients before their dental visits, the less chairtime will be needed to explain caries risk assessment and its benefits to them. In addition, the supplemental information given to them in the operatory will reinforce what they read earlier. Try to schedule and allow for the few more minutes it will require to explain caries risk assessment to patients and be able to answer their questions.
The benefit of having the entire team supporting the philosophy change is that the patients will hear it from more than one person and may actually require less of the dentist's direct time in education. However, the most effective message still has to come from the dentist. "Here is how we are changing and here is why" still needs to come from the doctor. The ultimate goal in the caries risk-assessment conversation with the patient is for them to understand that just treating their cavities will not treat their disease; cavities are only underlying signs and symptoms of the bacterial biofilm infection. They need to understand that the biofilm infection must be diagnosed and treated as a proper disease. They need to understand the concept of the balance between health and disease and the risk vs protective factors. With proper background, the patients should be able to help identify any changes in their risk factors during future visits. The more understanding and valid information patients have, the more capable they are of making good healthcare decisions for themselves. The more they know, the better decisions they make. It is always good to close the conversation asking them if the information shared with them makes sense and making sure they understand it. Almost universally, their response will be not only that it makes sense, but they wonder why no one has ever shared this information with them before.
STANDARD OF CARE ISSUES
Probably nothing causes more confusion or debate than discussions of standard of care issues. Most dentists have the mistaken belief that standard of care is determined by what "most" dentists are doing. Then there is the other reality that these standards are essentially established in courtrooms, or on the courthouse steps. It does not really matter what most dentists are doing or not doing, what matters is what the established science indicates a prudent dentist should be doing. But the standard of care issue is much more complex than that. There are regulatory issues involving state boards, and there are the changing standards being taught by the dental schools and tested by the national and state board exam. Even the ADA, in conjunction with the third party payment industry, contributes to the standards with the CDT codes. While there are a number of reasons to implement caries risk assessment into private practice, including legal and standard of care issues, the most important reason is the patient. As caregivers, we all respond to change and are motivated most when it is in the best interest of the people we serve.
CONCLUSION
Many private practices began practicing caries risk assessment a few years ago, when there was a wealth of scientific information and not much else for direction. There were no validated forms; there were no validated treatment regimens for treating the bacterial biofilm disease. Not surprisingly, this is uncomfortable territory for most dentists, as in the previous century the profession has depended on a one-size-fits-all approach to dental caries: surgically remove the cavity regardless of size or nature and replace it with an amalgam restoration. Now every patient must have his or her risk assessment evaluated individually, and every patient will be unique, and the treatment will need to be custom designed for each individual in the present tense. Then each patient must continually be monitored for treatment outcomes, and over the long term for recurrent signs of the disease process. A low-risk patient today may become a high-risk patient tomorrow. Assuredly, caries risk assessment, diagnosis, and treatment will continue to evolve and change as more clinical and scientific data is gathered in the future, but certainly this represents the best standard of care today. The bottom line is when a dental practice implements caries risk assessment, and an antimicrobial/probiotic approach to treating the disease on all patients, it is possible to control the disease process, provide improved treatment outcomes, restore the mouth to health, and maybe even prevent cavities before they start.
References
1. Clapp GW. The life and work of James Leon Williams. Chicago, Ill: The Dental Digest, The Dentist's Supply Company; 1925.
2. Black GV. Operative Dentistry. 1926.
3. Fejerskov O, Kidd E. Dental caries: The disease and its clinical management. Oxford University Press, UK: Blackwell Publishing; 2003.
4. Zickert I, Emilson CG, Krasse B. Streptococcus mutans, lactobacilli and dental health in 13-14 year old Swedish children. Community Dent Oral Epidemiol. 1982;10(2):77-81.
5. Zickert I, Emilson CG, Krasse B. Effect of caries preventive measures in children highly infected with the bacterium Streptococcus mutans. Arch Oral Biol. 1982;27(10):861-868.
6. Zickert I, Emilson CG, Krasse B. Microbial conditions and caries increment 2 years after discontinuation of controlled antimicrobial measures in Swedish teenagers. Community Dent Oral Epidemiol. 1987:15(5):241-244.
7. Florio FM, Klein MI, Pereira AC, et al. Time of initial acquisition of mutans streptococci by human infants. J Clin Pediatr Dent. 2004;28(4):303-308.
8. Caufield PW. Dental caries: and infectious and transmissible disease where have we been and where are we going? NY State Dent J. 2005;71(2):23-27.
9. Featherstone JD. The caries balance: contributing factors and early detection. J Calif Dent Assoc. 2003;31(2):129-134.
10. Merritt J, Anderson MH, Park NH, et al. Bacterial biofilm and dentistry. J Calif Dent Assoc. 2001;29(5):355-360.
11. Lappin-Scott HM, Costerton JW. Microbial Biofilms. Cambridge University Press. Cambridge UK. 2003.
12. Marsh PD. Host defenses and microbial homeostasis: role of microbial interactions. J Dent Res. 1989;68:1567-1575.
13. Busscher HJ, Evans LV. Oral Biofilms and Plaque Control. Philadelphia, Pa: Gordon and Breach Publishing; 1998.
14. Len AC, Harty DW, Jaques NA. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology. 2004;150:1339-1351.
15. Bradshaw DJ, McKee AS, Marsh PD. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989. 68(9)1298-1302.
16. Tenuta LMA, Ricomini Filho AP, Del Bel Cury AA, et al. Effect of sucrose on the selection of mutans streptococci and Lactobacilli in dental biofilm formed in situ. Caries Res. 2006;40(6):546-549.
17. Marsh PD. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14.
18. Young DA, Buchana P, Lubman RG, et al. CAMBRA is minimally invasive dentistry. Dental Products Report. 2006;40(3):42-45.
19. Kutsch VK, Kutsch CL. Manage caries: a minimally invasive approach. Dental Products Report. 2006;40(4):18-24.
20. Featherstone JD, Adair SM, Anderson MH, et al. Caries management by risk assessment: consensus statement, April 2002. J Calif Dent Assoc. 2003;31(3):257-269.
21. Walsh LJ. Dental plaque fermentation and its role in caries risk assessment. International Dentistry SA Australasian Edition. 2006;1(3):5-13.
22. Fontana M, Zero DT. Assessing patients' caries risk. J Am Dent Assoc. 2006;137(9):1231-1239.
23. DenBesten P, Berkowitz R. Early childhood caries: an overview with reference to our experience in California. J Calif Dent Assoc. 2003;31(2):139-143.
24. Bradshaw JD, Marsh PD. Analysis of pH driven disruption of oral microbial communities in vitro. Caries Res. 1998;32(6):456-462.
25. Maehara H, Iwami Y, Mayanagi H, et al. Synergistic inhibition by combination of fluoride and xylitol on glycolysis by mutans streptococci and its biochemical mechanism. Caries Res.2005;39(6):521-528.
26. Borutta A, Reuscher G, Hufnagl S, et al. Caries prevention with fluoride varnishes among preschool children. Gesundheitswesen. 2006;68(11):731-734.
27. Iijima Y, Cai F, Shen P, et al. Acid resistance of enamel subsurface lesions remineralized by a sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. Caries Res. 2004;38(6):551-556.
28. Tavss EA, Mellberg JR, Joziak M, et al. Relationship between dentifrice fluoride concentration and clinical caries reduction. Am J Dent. 2003;16(6):369-374.
29. Eckert R, He J, Yarbrough DK, et al. Targeted killing of Streptococcus mutans by a pheromone-guided "smart" antimicrobial peptide. Antimicrob Agents Chemother. 2006;-50(11):3651-3657.
30. Kutsch VK. How to integrate CAMBRA into private practice, part II. Doctor of Dentistry Oregon/SW Washington February 2004:9-10.