You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Adverse drug reactions are not uncommon, occurring in 10% to 20% of hospitalized patients and in approximately 7% of those in the ambulatory setting.1 By convention, adverse drug reactions are categorized as type A or type B. Type A reactions are most common and are caused by known pharmacological or toxic effects of a drug, such as nausea or diarrhea with antibiotics and tachycardia or palpitations with epinephrine. Type B reactions are less common and generally unpredictable. They include drug intolerance and idiosyncrasy but mostly embrace drug allergy and other events that resemble allergy, ie, pseudoallergy. It is important to distinguish these categories of drug reactions because patients often report any adverse event as an ‘‘allergy.’’
Pathogenesis of Allergy
Allergic reactions are immune mediated, generally triggered by lymphocytes. Gell and Coombs2 first categorized allergic reactions as class I through IV, based on distinct pathologic mechanisms (Table 1). This system still serves as a basic framework but has evolved into additional subcategories and more thorough explanations as our knowledge of immunology has continued to progress. For example, we know that allergic reactions may indeed occur in patients who have had no prior exposure to a drug. Formerly this was attributed solely to prior sensitization to a compound similar in molecular structure. Now pharmacologic interaction (the so-called p-i concept) offers an additional explanation in which subtle differences in receptors found on T memory cells and antigen-presenting cells enable binding and elicitation upon initial exposure.3,4 Nonetheless, it is still conventional to categorize the drug reactions addressed in this article as class I (immunoglobulin E [IgE] mediated) that have an immediate onset (generally within minutes to a few hours) or class IV (T-cell–mediated) that have a delayed onset (generally a day or more).
Regardless of the classification or precise mechanism, the pathogenesis of allergic reactions centers on the synthesis and release of chemical mediators.5 T lymphocytes mediate class IV reactions by secreting lymphokines that induce direct cytotoxic effects or activate macrophages and other lymphocytes to perform their functions. During class I reactions, B lymphocytes produce immunoglobulins, IgE in particular, that bind to mast cells and basophils. When antigen is introduced and attaches to these membrane-bound antibodies, the cells degranulate, releasing histamine and other mediators referred to collectively as autacoids. These mediators not only initiate immediate tissue responses but also recruit leukocytes that contribute to a late-phase response whose onset may be delayed for several hours. Mast cells are distributed throughout all connective tissues but are especially numerous beneath the skin and mucosal surfaces, including the upper and lower respiratory tract, where many clinical manifestations of allergic reactions occur.6
The degranulation of mast cells and basophils can be triggered by a variety of nonimmunologic mechanisms as well. For example, meperidine triggers mast cells to release preformed histamine, which produces localized signs and symptoms that are indistinguishable from true IgE-mediated allergic reactions. The term pseudoallergy has been adopted to distinguish reactions that do not have a proven immune mechanism.7 Reactions may be localized to tissues in the proximity of antigen exposure and include angioedema, urticaria, and contact dermatitis. Other reactions are more generalized and may be so severe as to involve multiple organ systems, leading to hypotension, bronchospasm, and laryngeal edema. Severe reactions are called anaphylaxis or anaphylactoid if an IgE mechanism has not been established. In truth, issues regarding allergic versus pseudoallergic reactions are more academic than pragmatic; signs and symptoms are attributed to the actions of various autacoids, not the specific mechanism for their release. An impressive number of autacoids have been identified, and they are summarized along with their functions in Table 2.
Patient Assessment and Incidence of Drug Allergy
A thorough medical history is standard of care before commencing any dental treatment. This includes thorough questioning regarding drug history, especially adverse reactions associated with drugs the dentist intends to administer or prescribe. Even though a patient reports an allergic reaction, this does not necessarily preclude the use of the particular drug or drug class in question. As mentioned previously, it is not uncommon for patients to label any adverse drug experience as an allergic reaction. When the history includes airway compromise or cutaneous reactions, allergy is more likely. In terms of cutaneous reactions, urticaria (hives) is most indicative of an IgE-mediated reaction, but the overwhelming majority of cutaneous reactions to drugs (~80%) are pruritus or rash, and these are not IgE mediated. Any potential for cross-reaction to similar agents is unlikely.8 Nevertheless, one should regard any of the following signs suspiciously: pruritus (itching), rash, urticaria (hives), or airway compromise. Local anesthetics, analgesics, and antibiotics are the most common drug classes used in dental practice, and allergic or pseudoallergic reactions have been reported for each.
Local Anesthetics
A patient’s claim for allergy to local anesthetics can be perplexing given the paramount importance of these agents in dental practice and the scarcity of alternative options available. Although the actual incidence of confirmed allergy to local anesthetics is extremely low (<1%), any claim must be given serious attention considering the staggering number of local anesthetic procedures we perform. There is little dispute that most adverse reactions involving local anesthetics are misstated as allergy. Syncopal episodes, including brief seizure-like activity, and cardiovascular events attributable to epinephrine should be ruled out by careful questioning and the patient reassured they do not represent allergic reactions. However, cutaneous reactions or airway compromise should be regarded as potentially allergic in nature. For these cases, an evaluation by an allergist is essential.
When referring a patient for allergy testing, it is wise to speak with the allergist and discuss providing cartridges of 3% mepivacaine, 4% prilocaine plain, or both. Also discuss the possibility of testing the patient for bisulfite sensitivity, so that formulations containing vasoconstrictors might also be used if the tests for the local anesthetics prove negative. (Formulations containing epinephrine will suppress any response and cannot be used for testing.) See Figure 1.
The common paradigm regarding local anesthetic allergy is that esters of para-aminobenzoic acid are the most common offenders and amide derivatives are rarely if ever implicated; instead, preservatives are blamed as the likely culprits. This thinking is no longer sound. Both allergic and pseudoallergic reactions to ester and amide local anesthetics, as well as methylparaben and metabisulfite preservatives, have been reported in the scientific literature.9-15 Neither is it acceptable to assume that cross-reactions do not occur among amide derivatives. Three unpublished case histories summarized in Table 3 illuminate these points.
In the rare event an allergist cannot identify an acceptable local anesthetic, one additional option is available to avoid a general anesthetic. Diphenhydramine (Benadryl) is an antihistamine that has demonstrated clinical efficacy in blocking sodium channels in peripheral nerves.16-19 It is not as effective as traditional local anesthetics, but has been used successfully following infiltration; evidence for efficacy following nerve block is less convincing. Diphenhydramine is very irritating to tissues and must be used as a 1% (10 mg/mL) concentration when used for this purpose. Even this concentration is more irritating than most local anesthetics. It is best to limit its use for single-tooth or localized soft-tissue procedures, injecting no more than 2 mL (20 mg) total. If a greater dose is used, consideration must be given to its sedative effects and an adult escort provided following the procedure. Duration of anesthesia is brief (~15–20 minutes), and if longer duration or additional sessions are planned, epinephrine can be added in the following manner. Purchase a 10-mL multidose vial of 1% diphenhydramine (10 mg/mL). Using a 1-mL tuberculin syringe, withdraw 1 mL and discard, leaving 9 mL diphenhydramine in the multidose vial. Now, using the same syringe, draw 0.1 mL (100 mcg) epinephrine from a 1:1000 formulation and dilute to 1 mL with normal saline. Inject this 1 mL (100 mcg) into the vial containing the remaining 9 mL of diphenhydramine. This will provide a 1:100,000 epinephrine concentration or 10 mcg/mL. For local anesthesia, use a 3-mL or 5-mL syringe with a 1-inch, 25 to 27-gauge needle to infiltrate 1-mL to 2-mL increments of the solution. Following the dental procedure, discard any unused medication.
Nonsteroidal Anti-Inflammatory Drugs
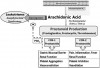
Nausea and dyspepsia (upset stomach) are the most common events labeled by patients as being allergic reactions to nonsteroidal anti-inflammatory drugs (NSAIDs), but a significant number of patients may describe reactions that appear allergic in nature. True IgE-mediated reactions to aspirin and NSAIDs have been confirmed, but they are rare, and the event is more often attributable to a pseudoallergic mechanism.7,20 The molecular structures of NSAIDs can be strikingly dissimilar, rendering cross IgE reactions unlikely, but all inhibit cyclooxygenases from converting arachidonic acid to prostaglandins. This shifts arachidonic acid metabolism toward another group of enzymes called lipoxygenases that convert arachidonic acid into leukotrienes. Even a subtle increase in these autacoids may lead to pseudoallergic reactions, especially in atopic patients (see Figure 2.). In the past, terms such as aspirin intolerance syndrome, aspirin-induced asthma, and aspirin triad have been used to describe this reaction. More recently, however, the various reactions to NSAIDs have been better characterized.
Aspirin-exacerbated respiratory disease is now the preferred term for NSAID-induced respiratory symptoms that occur in patients with underlying asthma, rhinitis, or sinusitis.7 This reaction is believed to be related to heritable alterations in arachidonic acid metabolism that enhance leukotriene synthesis when exposed to drugs that inhibit cyclooxygenase-1 enzymes. In these patients, all conventional NSAIDs should be avoided, but selective cyclooxygenase-2 inhibitors such as celecoxib (Celebrex) can be taken safely.7 Considerations are identical for patients who suffer from chronic idiopathic urticaria and experience exacerbation when taking an NSAID. For patients having no underlying respiratory or cutaneous disease, a history of urticaria, angioedema, or anaphylaxis following NSAID exposure is more suggestive of an actual IgE-mediated reaction. For these patients, an alternate NSAID may be tolerated. NSAIDs belong to several unique molecular classifications, and if the reaction was truly IgE mediated, there is no cross-reactivity with those derived from a different class7 (see Table 4). For example, a patient reacting to a propionic acid derivative such as ibuprofen or naproxen will likely tolerate diclofenac, an acetic acid, or diflunisal, a salicylic acid.
Despite these illuminations, complete details for patients claiming adverse reactions are often imprecise, and it may be more sensible to simply adopt a cautious protocol. For patients describing only a history of rash or pruritus, it is safe to select an alternative NSAID. For those describing urticaria or respiratory symptoms, it is prudent to avoid all NSAIDs and prescribe acetaminophen, regardless of their underlying respiratory status. It should be mentioned that true IgE-mediated reactions to acetaminophen have also been reported, but there is no connection to reactions involving NSAIDs.21
Opioids
The most common adverse reaction to opioids is nausea, and this is almost always the issue in patients claiming allergy. Only one case of IgE-mediated reaction has been published in the literature, and this claimed cross-reaction among several opioids, including codeine.22 However, nearly all opioids are capable of inducing pseudoallergic reactions by triggering degranulation of mast cells and the direct release of histamine.23,24 (Fentanyl and its derivatives are notable exceptions.) For this reason allergy testing is problematic. (See case 1 in Table 3.) Opioid-induced pseudoallergic reactions are rarely life threatening, and if non-opioids cannot control pain, graded doses of a non-implicated opioid may be tried.7
Antibiotics
The penicillins and cephalosporins are the most commonly used antibiotics in dental practice. Both have been confirmed as producing allergic and pseudoallergic reactions, but the actual incidence is well overstated.25,26
As many as 1 in 10 patients report a history of allergy to penicillin, but up to 90% of these are able to tolerate penicillin and are designated ‘‘penicillin allergic’’ unnecessarily.7 Most patients claiming history of allergy to penicillin can tolerate cephalosporins.27-29 One might also consider the time elapsed since the reaction occurred. It is not unusual for adults to offer a vague history of reaction as a child. Approximately 50% of patients with actual IgE reactions to penicillin lose their sensitivity after 5 years, and this increases to 80% after 10 years.28,30
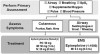
Issues regarding the potential for cross-allergenicity between penicillins and cephalosporins were formerly thought related to the beta-lactam ring, but recent evidence has established that they are related more to similarities in the R side chains.31,32 It is generally accepted that patients having a history of IgE-mediated reaction to a penicillin should be managed using a non–beta-lactam antibiotic.33 Urticaria (hives) is IgE mediated but accounts for only 10% of all exanthematous drug reactions. The overwhelming majority of cutaneous reactions to penicillins are pruritus or rash, and these are not IgE mediated. Any potential for cross-reaction is unlikely.7,8 Furthermore, upon additional questioning, most patients claiming allergy to penicillin are discovered to have experienced stomach upset (dyspepsia), nausea, or diarrhea. Although macrolides and clindamycin are conventionally considered the alternatives of choice in patients allergic to penicillins, the macrolides have become less attractive, as addressed in a previous continuing education article in this journal.34 It is preferable to substitute an alternate penicillin or cephalosporin for a patient claiming penicillin allergy, provided the nature of the reaction was merely pruritic (itch) or a maculopapular rash. A history of urticaria (hives) or anaphylactoid symptoms are more convincing evidence that the patient’s reaction to penicillin was truly IgE mediated, and in this case, there is little recourse but to refrain from prescribing any beta-lactam derivative (see Figure 3).
Allergic or pseudoallergic reactions to other classes of antibiotics used in dentistry are more uncommon and less understood. Nonetheless, clinical reports of such reactions do appear in the literature.35-37 Simply stated, a patient’s claim of a cutaneous reaction or airway compromise leaves little recourse but to avoid prescribing the offending drug.
Latex
Natural rubber latex is a milky white sap obtained from rubber trees (Hevea brasiliensis) and is used in over 40,000 medical products.38,39 The allergenicity of this substance was first published in 1979, and its incidence began to rise during the latter 1980s and 1990s in concert with improved emphasis on infection control in response to the AIDS epidemic.40,41 Since then, the incidence has declined as healthcare providers have become well informed and are knowledgeable in providing a latex-free environment.41,42
Although most dental practices now avoid latex-containing materials, issues are somewhat cloudy regarding any latex content of the rubber stoppers and diaphragms found in drug vials and anesthetic cartridges. To address this issue, publications from 1966 to 2001 were reviewed by Shojaei and Haas.43 They found ample evidence of reactions related to latex in medication vials, but there were no reports related to any latex content of local anesthetic cartridges. Although this is reassuring and any risk is certainly remote, it cannot be a foregone conclusion that cartridges carry no risk. When managing a patient with a history of a severe reaction to latex, it would be wise to consult the specific anesthetic manufacturer regarding the latex content of the product.
Management of Allergic Reactions
Clinical manifestations for either allergic or pseudoallergic reactions are identical, and their distinction is irrelevant. It is more useful to categorize reactions as minor or major. Those confined to the skin are minor and are not life threatening, whereas those involving the upper and lower airway are major and may be life threatening. Cutaneous reactions include pruritus (itch), rash, and urticaria (hives). These exanthems are generally mediated by histamine and can be managed using an antihistamine such as diphenhydramine (Benadryl). It can be administered as 25-mg to 50-mg by intramuscular (IM) injection in the deltoid muscle using a 50-mg/mL concentration (0.5-mL to 1.0 mL). It is generally recommended that drug volumes administered at this site be limited to 1 mL.44,45 The identical dose range may be administered intravenously, but the concentration should be diluted to 10 mg/mL because of its irritating properties.
Major or anaphylactoid reactions involve the respiratory tract and in severe cases the cardiovascular system. These reactions are mediated by autacoids other than histamine, and there are no immediate-acting antagonists for these particular mediators. Their influence must be countered physiologically by effects provided by epinephrine.46,47 Subcutaneous injection is no longer the preferred route for emergency epinephrine administration. Subcutaneous vasculature contains only alpha receptors, and epinephrine produces vasoconstriction, delaying its absorption. Muscle vasculature is populated with greater numbers of beta-2 receptors, and epinephrine dilates these vessels, thereby speeding absorption.
The conventionally recommended site for IM injection is the vastus lateralis. This is based largely on a study published by Simons et al48 that studied serum levels of epinephrine following subcutaneous and IM injections into deltoid and vastus lateralis muscles. Curiously, they found serum levels were strikingly lower following administrations at the deltoid site, but subcutaneous injection at this location actually led to a slightly higher serum level than IM injection. In contrast, thigh injections were administered only IM. Other studies assessing blood flow among various muscles and absorption of other medications have found the deltoid muscle superior.49-51 This point is further supported by the deltoid muscle’s being the preferred site for hepatitis B vaccination. Certainly the vastus lateralis is the preferred site for autoinjection by patients using the EpiPen because of its accessibility. Likewise, for children the deltoid muscle may not be adequate in size. However, both muscles are richly perfused and allow adequate rates of absorption. The site selected should be based on ease of access.
The popularity of EpiPens is another consideration. They are commonly included in office emergency kits, but they are very expensive to maintain. Costs range from $150 to $200 each for both child (0.15 mg) and adult (0.3 mg) formulations, and their shelf life is only 2 years. For those experienced in intravenous sedation and general anesthesia, the use of single-dose vials and ampules is routine and is far more cost-effective. For those practitioners inexperienced with preparation of injectables, the EpiPen is more advisable. Beneficial effects and dosages for epinephrine are summarized in Table 5.
In order of their frequency, anaphylactoid reactions include tongue swelling and laryngeal edema, bronchospasm, and hypotension. The swelling of laryngeal mucosa, as well as neighboring pharyngeal mucosa and tongue, will generally present as stridor or high-pitched crowing sounds during inspiration. The conscious patient will grasp his or her throat and complain of throat tightness or tongue swelling. The alpha-receptor agonist action of epinephrine will constrict submucosal vessels and reduce swelling.
Bronchospasm is a lower-airway obstruction due to contraction or spasm of bronchial smooth muscle. It may be a consequence of a hyperreactive airway typical in asthmatic patients or the result of an anaphylactoid reaction, independently or in combination with laryngeal edema. Regardless of the cause for bronchospasm, the patient will exhibit dyspnea and wheezing attributed to obstruction in the chest, not the throat or mouth. The beta-2 agonist action of epinephrine will relax bronchial smooth muscle. In the event bronchospasm is the sole complication, a more selective beta-2 agonist such as albuterol is preferred over epinephrine because it is less likely to produce positive cardiotonic side effects attributed to stimulation of cardiac beta-1 receptors. Albuterol is administered via metered inhaler as two to three activations but requires patients to cooperate if it is to be administered effectively. Spacer chambers can be attached to inhalers and minimize the need for a coordinated effort on the part of the patient. However, if a patient becomes hysterical, or for other reasons cannot be administered an inhalant, epinephrine injection should be administered.
Severe anaphylactoid reactions may also lead to hypotension that will likewise be countered by epinephrine. Activation of alpha receptors on veins produces venoconstriction improving venous return (preload) and beta-1 receptor activation increases myocardial contractility. Both actions improve cardiac output and systolic blood pressure. At conventional doses, epinephrine may not markedly increase arterial resistance and diastolic blood pressure because of its beta-2 receptor action on arteries. If intravenous access is available, fluid infusion is also encouraged.
Additional agents mentioned frequently in dental literature for managing asthma, allergic, or anaphylactoid reactions include aminophylline and corticosteroids. These are not recommended for initial acute treatment because of limited efficacy, significant toxicity (aminophylline), or delayed onset, eg, several hours for corticosteroids. An algorithm for the management of acute allergic reactions is presented in Figure 4. The conventional dose for epinephrine is 0.3 mg (0.15 mg for children) of a 1:1000 concentration administered by IM injection. This is recommended even if intravenous access is available. Intravenous titration of 0.1-mg increments using a 1:10,000 concentration should be reserved for extremely severe or refractory cases accompanied by profound hypotension.47,52,53
References
1. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005;5:309-316.
2. Gell PGH, Coombs RRA. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In Gell PGH, Coombs RRA, Hachmann PJ, eds. Clinical Aspects of Immunology. 3rd ed. Oxford, United Kingdom: Blackwell Scientific; 1975.
3. Hausmann O, Schnyder B, Pichler WJ. Etiology and pathogenesis of adverse drug reactions. Chem Immunol Allergy. 2012;97:32-46.
4. Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84:268-272.
5. Levinson W. Hypersensitivity (allergy). In: Levinson W. Review of Medical Microbiology and Immunology. 12th ed. New York, NY: McGraw-Hill Companies Inc: 2012.
6. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257-265.
7. Solensky R, Kahn DA. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259-273.
8. Stern RS. Clinical practice. Exanthematous drug eruptions. N Engl J Med. 2012;366:2492-2501.
9. Schatz M. Adverse reactions to local anesthetics. Immunol Allergy Clin North Am. 1992;12:585-609.
10. Schwartz HJ, Gilbert IA, Lenner KA, et al. Metabisulfite sensitivity and local dental anesthesia. Ann Allergy. 1989;62:83-86.
11. Bhole MV, Manson AL, Seneviratne SL, Misbah SA. IgE-mediated allergy to local anaesthetics: separating fact from perception: a UK perspective. Br J Anaesth. 2012;108:903-911.
12. Speca SJ, Boynes SG, Cuddy MA. Allergic reactions to local anesthetic formulations. Dent Clin North Am. 2010;54:655-664.
13. Gall H, Kaufmann R, Kalveram CM. Adverse reactions to local anesthetics: analysis of 197 cases. J Allergy Clin Immunol. 1996;97:933-937.
14. Gonzalez-Delgado P, Anton R, Soriano V, et al. Cross-reactivity among amide-type local anesthetics in a case of allergy to mepivacaine. J Investig Allergol Clin Immunol. 2006;16:311-313.
15. Venemalm L, Degerbeck F, Smith W. IgE-mediated reaction to mepivacaine. J Allergy Clin Immunol. 2008;121:1058-1059.
16. Gallo WJ, Ellis E III. Efficacy of diphenhydramine hydrochloride for local anesthesia before oral surgery. J Am Dent Assoc. 1987;115:263-266.
17. Willett J, Reader A, Drum M, et al. The anesthetic efficacy of diphenhydramine and the combination diphenhydramine/lidocaine for the inferior alveolar nerve block. J Endod. 2008;34:1446-1450.
18. Pavlidakey PG, Brodell EE, Helms SE. Diphenhydramine as an alternative local anesthetic agent. J Clin Aesthet Dermatol. 2009;2:37-40.
19. Dire DJ, Hogan DE. Double-blinded comparison of diphenhydramine versus lidocaine as a local anesthetic. Ann Emerg Med. 1993;22:1419-1422.
20. Babu KS, Salvi SS. Aspirin and asthma. Chest. 2000;118:1470-1476.
21. Rutkowski K, Nasser SM, Ewan PW. Paracetamol hypersensitivity: clinical features, mechanism and role of specific IgE. Int Arch Allergy Immunol. 2012;159:60-64.
22. Harle DG, Baldo BA, Coroneos NJ, et al. Anaphylaxis following administration of papaveretum. Case report: implication of IgE antibodies that react with morphine and codeine and identification of an allergenic determinant. Anesthesiology. 1989;71:489-494.
23. Flacke JW, Flacke WE, Bloor BC, et al. Histamine release by four narcotics: a double-blind study in humans. Anesth Analg. 1987;66:723-730.
24. Weiss ME, Adkinson NF, Hirshman CA. Evaluation of allergic drug reactions in the perioperative period. Anesthesiology. 1989;71:483-486.
25. Erffmeyer JE. Reactions to antibiotics. Immunol Allergy Clin North Am. 1992;12:633-648.
26. Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Ann Intern Med. 2000;160:2819-2822.
27. Romano A, Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004;141:16–22.
28. Goodman EJ, Morgan MJ, Johnson PA, et al. Cephalosporins can be given to penicillin-allergic patients who do not exhibit an anaphylactic response. J Clin Anesth. 2001;13:561-564.
29. Daulat SB, Solensky R, Earl HS, et al. Safety of cephalosporin administration to patients with histories of penicillin allergy. J Allergy Clin Immunol. 2004;113:1220-1222.
30. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103:918-924.
31. Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: a literature review. J Emerg Med. 2012;42:612-620.
32. Solensky R. Allergy to beta-lactam antibiotics. J Allergy Clin Immunol. 2012;130:1442.
33. Abramowicz M, ed. Cephalosporins for patients with penicillin allergy. Med Lett Drugs Ther. 2012;54:101.
34. Becker DE. Antimicrobial drugs. Anesth Prog. 2013;60:111-122.
35. Asensio Sanchez T, Davila I, Moreno E, et al. Anaphylaxis due to metronidazole with positive skin prick test. J Investig Allergol Clin Immunol. 2008;18:138-139.
36. Nakamura Y, Watamatsu K, Muto M. Drug-induced hypersensitivity syndrome induced by clindamycin. Acta Derm Venereol. 2013;93:83-84.
37. Tian D, Mohan RJ, Stallings G. Drug rash with eosinophilia and systemic symptoms syndrome associated with clindamycin. Am J Med. 2010;123:e7-e8.
38. Slater JE. Allergic reactions to natural rubber. Ann Allergy. 1992;68:203-211.
39. Warpinski JR, Folgert J, Cohen M, et al. Allergic reaction to latex: a risk factor for unsuspected anaphylaxis. Allergy Proc. 1991;12:95-102.
40. Nutter AF. Contact urticaria to rubber. Br J Dermatol. 1979;101:597-598.
41. Ranta PM, Ownby DR. A review of natural-rubber latex allergy in health care workers. Clin Infect Dis. 2004;38:252-256.
42. Hepner DL, Castells MC. Latex allergy: an update. Anesth Analg. 2003;96:1219-1229.
43. Shojaei AR, Haas DA. Local anesthetic cartridges and latex allergy: a literature review. J Can Dent Assoc. 2002;68:622-626.
44. Beyea SC, Nicoll LH. Administration of medications via the intramuscular route: an integrative review of the literature and research-based protocol for the procedure. Appl Nurs Res. 1995;8:23-33.
45. Berman A, Snyder SJ, eds. Kozier’s and Erb’s Fundamentals of Nursing: Concepts, Process and Practice. 9th ed. Upper Saddle River, NJ: Pearson Education, Inc; 2012:888-890.
46. Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
47. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S829-S861.
48. Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108:871-873.
49. Evans EF, Proctor JD, Fratkin MJ, et al. Blood flow in muscle groups and drug absorption. Clin Pharmacol Ther. 1975;17:44-47.
50. Korttila K, Linnoila M. Absorption and sedative effects of diazepam after oral administration and intramuscular administration into the vastus lateralis muscle and the deltoid muscle. Br J Anaesth. 1975;47:857-862.
51. Sullivan KJ, Berman LS, Koska J, et al. Intramuscular atropine sulfate in children: comparison of injection sites. Anesth Analg. 1997;84:54-58.
52. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.e1–442.
53. Tran TP, Muelleman RL. Allergy, Hypersensitivity and Anaphylaxis. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, Pa: Mosby Elsevier; 2009.